18805
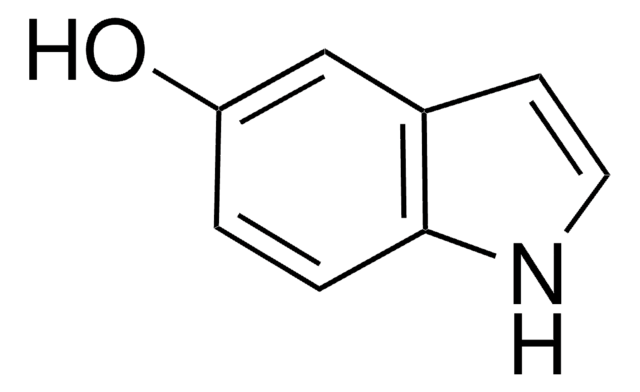
6-Hydroxyindole
≥99.0% (GC)
Sinónimos:
6-Indolol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H7NO
Número de CAS:
Peso molecular:
133.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥99.0% (GC)
form
solid
mp
126-132 °C
SMILES string
Oc1ccc2cc[nH]c2c1
InChI
1S/C8H7NO/c10-7-2-1-6-3-4-9-8(6)5-7/h1-5,9-10H
InChI key
XAWPKHNOFIWWNZ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- Reactant for preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Reactant for asymmetrical synthesis of notoamide J as a potential biosynthetic precursor of prenylated indole alkaloids
- Reactant for preparation of (quinolinyloxymethyl)isoxazolecarboxylate esters antituberculosis agents
- Reactant for preparation of indolyl(propanolamine) derivatives as HIV inhibitors
- Reactant for preparation of indoleoxyacetic acid derivatives as peroxisome proliferator-activated receptor agonists
- Reactant for preparation of 1-aroylindole 3-aroylindoles combretastatin A-4 analogs as antitumor agents and tubulin polymerization inhibitors
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Jintae Lee et al.
Applied and environmental microbiology, 73(13), 4100-4109 (2007-05-08)
Since indole is present at up to 500 microM in the stationary phase and is an interspecies biofilm signal (J. Lee, A. Jayaraman, and T. K. Wood, BMC Microbiol. 7:42, 2007), we investigated hydroxyindoles as biofilm signals and found them
Damon Borg et al.
Journal of analytical toxicology, 41(1), 6-16 (2016-09-30)
Synthetic cannabinoids are a group of psychoactive compounds that mimic the effects of Δ9-tetrahydrocannabinol, the primary psychoactive constituent of marijuana (Cannabis sativa L). The Drug Enforcement Administration has classified many of the most common cannabinoids as Schedule 1 controlled substances.
Stefan W Toennes et al.
Drug testing and analysis, 10(4), 644-650 (2017-10-03)
Each year, synthetic cannabinoids occur in high numbers on the illicit drug market, but data on their detectability are rarely available. A pilot study was performed to assess adverse effects of JWH-018, which is one of the oldest and best
Martin Švidrnoch et al.
Talanta, 150, 568-576 (2016-02-04)
Perfluoroheptanoic acid was employed as a volatile micellar phase in background electrolyte for micellar electrokinetic chromatography-tandem mass spectrometry separation and determination of 15 selected naphthoyl- and phenylacetylindole- synthetic cannabinoids and main metabolites derived from JWH-018, JWH-019, JWH-073, JWH-200 and JWH-250.
Yoshimitsu Yamazaki et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 65(1-2), 49-54 (2010-04-02)
A recent study showed that N-acylserotonin derivatives have strong inhibitory activity against tyrosinase. To clarify the role of the 5-hydroxy group in the indole ring, 2-, 4-, 5-, 6-, and 7-hydroxyindole and 11 related compounds such as 5-hydroxyindan and 6-hydroxyquinoline
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico