174823
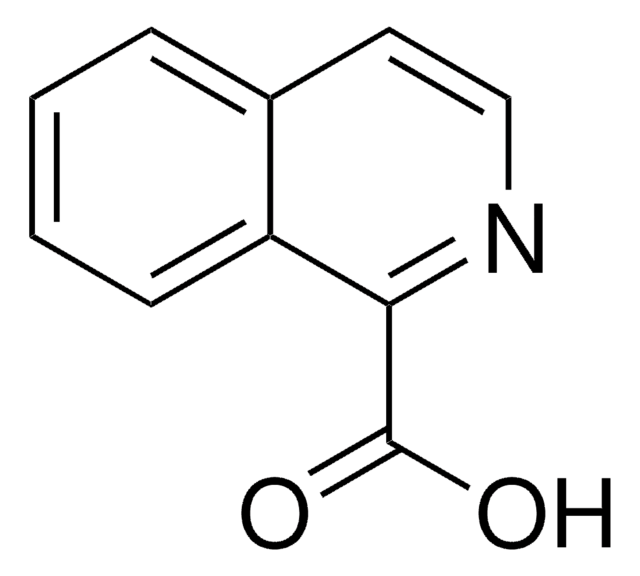
4-Quinolinecarboxylic acid
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H7NO2
Número de CAS:
Peso molecular:
173.17
Beilstein/REAXYS Number:
5224
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
mp
254-255 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1ccnc2ccccc12
InChI
1S/C10H7NO2/c12-10(13)8-5-6-11-9-4-2-1-3-7(8)9/h1-6H,(H,12,13)
InChI key
VQMSRUREDGBWKT-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
4-Quinolinecarboxylic acid was used in the coupling reaction with diamine linker. A 4-quinolinecarboxylic acid analogue, brequinar sodium was used to inhibit dihydroorotate dehydrogenase and the de novo biosynthesis of pyrimidine.
Biochem/physiol Actions
4-Quinolinecarboxylic acid showed anti-tumor activity against L1210 leukemia and B16 melanoma.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Afshin Zarghi et al.
Bioorganic & medicinal chemistry, 17(14), 5312-5317 (2009-06-30)
A group of 4-carboxyl quinoline derivatives possessing a methylsulfonyl COX-2 pharmacophore at the para position of the C-2 phenyl ring were designed and synthesized as selective COX-2 inhibitors. In vitro COX-1/COX-2 structure-activity relationships were determined by varying the substituents on
The cardiovascular and respiratory effects of 3-hydroxy-2-phenyl cinchoninic acid.
H A WALKER et al.
The Journal of pharmacology and experimental therapeutics, 102(2), 71-78 (1951-06-01)
7-Methyl-thiocinchoninamide.
A G RENFREW
Journal of the American Pharmaceutical Association. American Pharmaceutical Association, 40(9), 467-467 (1951-09-01)
Dorothée Duvelleroy et al.
Organic & biomolecular chemistry, 3(20), 3794-3804 (2005-10-08)
Rapid synthesis of quinoline-4-carboxylic acid derivatives has been achieved by reaction of 2-methoxy acrylates or acrylamides with N-arylbenzaldimines in acetonitrile under InCl3 catalysis and microwave irradiation. Isolated yields up to 57% within 3 min have been obtained. The Lewis acid
M-J Chen et al.
European review for medical and pharmacological sciences, 23(10), 4360-4367 (2019-06-08)
The aim of this work was to explore whether lncRNA-MEG3 could serve as a serum biomarker for diagnosing chronic hepatitis B (CHB) and improve the early diagnostic and treatment efficacies. Serum level of lncRNA-MEG3 in CHB patients and healthy controls
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico