146498
Trimethyltin chloride
Sinónimos:
Chlorotrimethylstannane
About This Item
Productos recomendados
form
crystals
Quality Level
mp
37-39 °C (lit.)
SMILES string
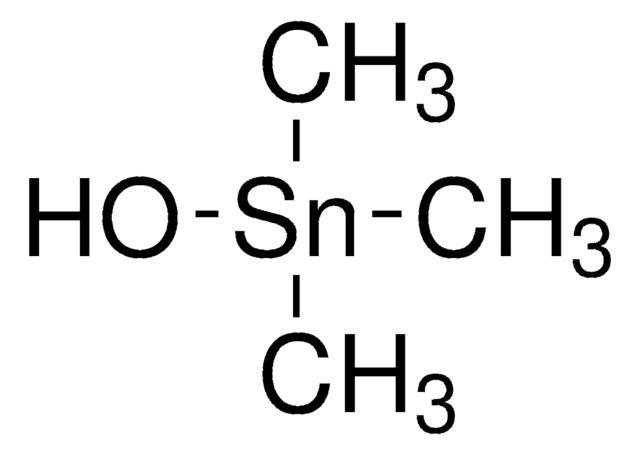
C[Sn](C)(C)Cl
InChI
1S/3CH3.ClH.Sn/h3*1H3;1H;/q;;;;+1/p-1
InChI key
KWTSZCJMWHGPOS-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
It can also be used as a reagent to prepare:
- Organotrimethyltin derivatives by reacting with organocopper compounds via transmetalation reaction.
- Acetophenone by palladium-catalyzed coupling reaction with benzoyl chloride.
- Optically active propargyl trimethylstannane by treating with chiral allenyltitanium.
- Trimethylstannyl nucleophiles, which are applicable in the formation of Sn-C bonds via SN2 reactions, SRN1 reactions, and halogen-metal exchanges.
- Carbocycles by reacting with unactivated dienes or trienes via radical-mediated carbocyclization reaction in the presence of NaBH3CN and a catalytic amount of AIBN.
Me3SnCl can also be used as a Lewis acid catalyst in asymmetric allylic alkylation reactions.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 2
flash_point_f
206.6 °F - closed cup
flash_point_c
97 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico