78825
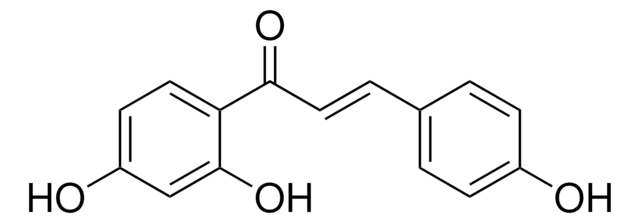
Liquiritigenin
≥97.0% (HPLC)
Sinónimos:
7,4′-Dihydroxyflavanone, 7-Hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one
About This Item
Productos recomendados
Ensayo
≥97.0% (HPLC)
Formulario
powder or crystals
impurezas
≤7% water
aplicaciones
metabolomics
vitamins, nutraceuticals, and natural products
cadena SMILES
Oc1ccc(cc1)[C@@H]2CC(=O)c3ccc(O)cc3O2
InChI
1S/C15H12O4/c16-10-3-1-9(2-4-10)14-8-13(18)12-6-5-11(17)7-15(12)19-14/h1-7,14,16-17H,8H2/t14-/m0/s1
Clave InChI
FURUXTVZLHCCNA-AWEZNQCLSA-N
Descripción general
Aplicación
- to study its inhibitory effect on tumor metastasis in the treatment of colorectal cancer
- as a reference standard for ultra-performance liquid chromatography (UPLC) of Chaihu-Shugan-San (CSS) extract
- as a potential antiviral drug against hepatitis C virus (HCV) infection
Acciones bioquímicas o fisiológicas
Envase
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico