68783
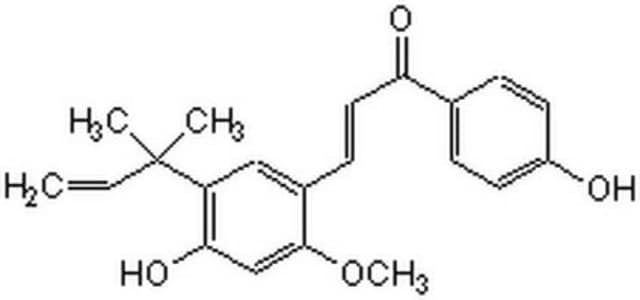
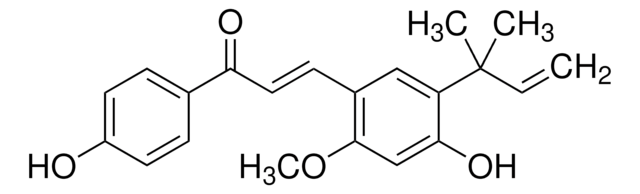
Licochalcone A
≥96.0% (HPLC)
Sinónimos:
(E)-3-[5-(1,1-Dimethyl-2-propenyl)-4-hydroxy-2-methoxyphenyl]-1-(4-hydroxyphenyl)-2-propen-1-one, 4′,4-Dihydroxy-3-α,α-dimethylallyl-6-methoxychalcone
About This Item
Productos recomendados
Ensayo
≥96.0% (HPLC)
Formulario
powder
temp. de almacenamiento
−20°C
cadena SMILES
COc1cc(O)c(cc1\C=C\C(=O)c2ccc(O)cc2)C(C)(C)C=C
InChI
1S/C21H22O4/c1-5-21(2,3)17-12-15(20(25-4)13-19(17)24)8-11-18(23)14-6-9-16(22)10-7-14/h5-13,22,24H,1H2,2-4H3/b11-8+
Clave InChI
KAZSKMJFUPEHHW-DHZHZOJOSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Acciones bioquímicas o fisiológicas
Envase
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico