P-075
Primidone solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Productos recomendados
grado
certified reference material
Nivel de calidad
Formulario
liquid
Características
Snap-N-Spike®/Snap-N-Shoot®
envase
ampule of 1 mL
fabricante / nombre comercial
Cerilliant®
concentración
1.0 mg/mL in methanol
técnicas
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
aplicaciones
forensics and toxicology
Formato
single component solution
temp. de almacenamiento
−20°C
cadena SMILES
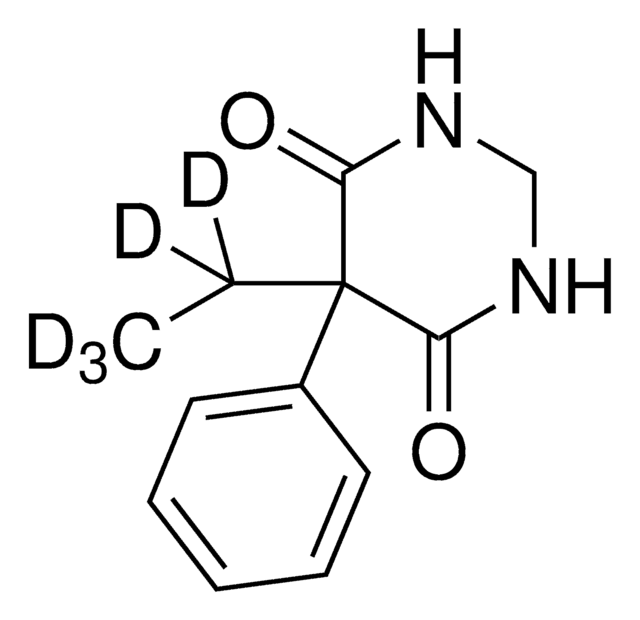
CCC1(C(=O)NCNC1=O)c2ccccc2
InChI
1S/C12H14N2O2/c1-2-12(9-6-4-3-5-7-9)10(15)13-8-14-11(12)16/h3-7H,2,8H2,1H3,(H,13,15)(H,14,16)
Clave InChI
DQMZLTXERSFNPB-UHFFFAOYSA-N
Información sobre el gen
human ... GABRA1(2554) , GABRA2(2555) , GABRA3(2556) , GABRA4(2557) , GABRA5(2558) , GABRA6(2559) , GABRB1(2560) , GABRB2(2561) , GABRB3(2562) , GABRD(2563) , GABRE(2564) , GABRG1(2565) , GABRG2(2566) , GABRG3(2567) , GABRP(2568) , GABRQ(55879) , SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
Descripción general
Aplicación
- LC-MS/MS-Based Quantification of 9 Antiepileptic Drugs From a Dried Sample Spot Device.: This study utilizes PrimiDonesolution as a reagent in the development of a high-throughput method for quantifying antiepileptic drugs. PrimiDone is critical for accurate measurements in biochemical and pharmacological research, especially in epilepsy studies (DUrso et al., 2019).
- Application of packed column supercritical fluid chromatography to the simultaneous determination of seven anticonvulsant drugs.: The research demonstrates the use of PrimiDonesolution in supercritical fluid chromatography to simultaneously determine multiple anticonvulsants, highlighting its importance as a stable reagent for laboratory use and anticonvulsant research (Bhoir et al., 1999).
- The role of technical, biological and pharmacological factors in the laboratory evaluation of anticonvulsant drugs. I. The influence of administration vehicles.: This paper discusses the use of PrimiDonesolution as a vehicle in laboratory evaluations of anticonvulsant drugs, emphasizing its stability and reliability for pharmacological experimentation and neurological disorder research (Loscher et al., 1990).
- Simple GLC analysis of anticonvulsant drugs in commercial dosage forms.: This study utilizes PrimiDonesolution in gas-liquid chromatography to analyze commercial anticonvulsant drugs, underlining its role as a high-purity reagent for biochemical studies and anticonvulsant research (Watson et al., 1978).
- Rapid micromethod for measuring anticonvulsant drugs in serum by high-performance liquid chromatography.: PrimiDonesolution is used in a rapid micromethod for serum analysis of anticonvulsants, demonstrating its utility as a PrimiDone reagent for anticonvulsant research and in vitro biochemical studies (Soldin and Hill, 1976).
Información legal
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Órganos de actuación
Eyes,Central nervous system
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
49.5 °F - closed cup
Punto de inflamabilidad (°C)
9.7 °C - closed cup
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico