684031
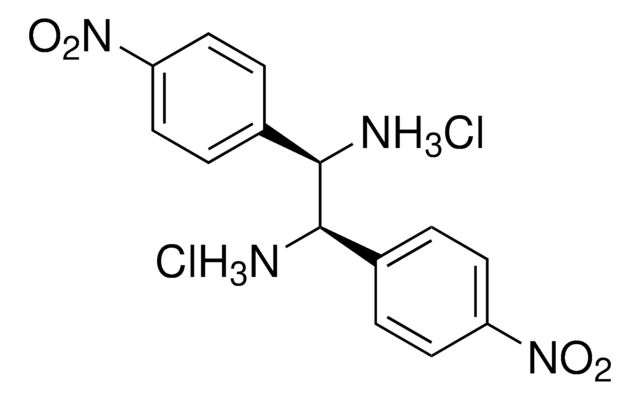
(1S, 2S)-1,2-Bis(4-nitrophenyl)ethylenediamine dihydrochloride
97%
Sinónimos:
(1S, 2S)-1,2-Bis(4-nitrophenyl)-1,2-ethanediamine dihydrochloride
About This Item
Productos recomendados
assay
97%
form
powder
optical activity
[α]22/D -84.0°, c = 1 in H2O
mp
202-207 °C
SMILES string
Cl.Cl.N[C@H]([C@@H](N)c1ccc(cc1)[N+]([O-])=O)c2ccc(cc2)[N+]([O-])=O
InChI
1S/C14H14N4O4.2ClH/c15-13(9-1-5-11(6-2-9)17(19)20)14(16)10-3-7-12(8-4-10)18(21)22;;/h1-8,13-14H,15-16H2;2*1H/t13-,14-;;/m0../s1
InChI key
PDPYGNJVCKPVGM-AXEKQOJOSA-N
Application
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Chiral vicinal diamines are of tremendous interest to the synthetic chemist as they are found in many chiral catalysts and pharmaceuticals.
Contenido relacionado
The Chin group is interested in computational and experimental approaches to understanding stereoselective recognition and catalysis. Their studies in weak forces (H-bonding, electronic and steric effects) has led to a highly efficient method for making limitless varieties of chiral vicinal diamines from the 'mother diamine' that are useful for developing stereoselective organocatalysts or transition metal-based catalysts as well as for developing drugs (Acc Chem Res (2012) p1345). The 'mother diamine' is also useful for making binol, monophos and binap analogs. The Chin group is also interested in using reversible covalent bonds for stereoselective recognition and L to D conversion of natural and non-natural amino acids (EJOC (2012) p229).
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico




![(1R,2R)-(+)-N,N′-Dimethyl-1,2-bis[3-(trifluoromethyl)phenyl]ethanediamine 97%](/deepweb/assets/sigmaaldrich/product/structures/408/938/05de1ba4-8e30-49a7-996e-99aa9340a1f4/640/05de1ba4-8e30-49a7-996e-99aa9340a1f4.png)

