669032
PEPPSI™-IPr catalyst
98%, Umicore
Sinónimos:
[1,3-Bis(2,6-Diisopropylphenyl)imidazol-2-ylidene](3-chloropyridyl)palladium(II) dichloride
About This Item
Productos recomendados
Quality Level
assay
98%
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
manufacturer/tradename
Umicore
mp
230 °C
SMILES string
Cl[Pd]Cl.Clc1cccnc1.CC(C)c2cccc(C(C)C)c2N3CN(C=C3)c4c(cccc4C(C)C)C(C)C
InChI
1S/C27H38N2.C5H4ClN.2ClH.Pd/c1-18(2)22-11-9-12-23(19(3)4)26(22)28-15-16-29(17-28)27-24(20(5)6)13-10-14-25(27)21(7)8;6-5-2-1-3-7-4-5;;;/h9-16,18-21H,17H2,1-8H3;1-4H;2*1H;/q;;;;+2/p-2
InChI key
BLDKGTGQENJFON-UHFFFAOYSA-L
General description
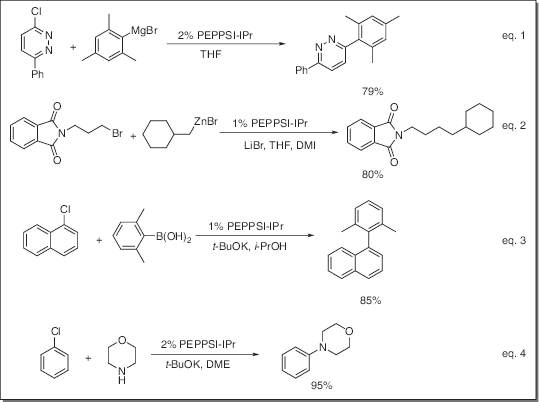
Application
- Catalyst for Kumada-Tamao-Corriu (KTC) reaction (eq. 1)
- Catalyst for Negishi coupling reaction (eq. 2)
- Catalyst for Suzuki coupling reaction (eq. 3)
- Catalyst for Buchwald-Hartwig amination reaction (eq. 4)
For small scale and high throughput uses, product is also available as ChemBeads (931063)
Legal Information
This product, its manufacturing or use, is the subject of one or more issued or pending U.S. Patents (and foreign equivalents) owned or controlled by Umicore PMC. The purchase of this product from Umicore PMC through Sigma-Aldrich, its affiliates or their authorized distributors conveys to the buyer a limited, one-time, non-exclusive, non-transferable, non-assignable license. Buyer′s use of this product may infringe patents owned or controlled by third parties. It is the sole responsibility of buyer to ensure that its use of the product does not infringe the patent rights of third parties or exceed the scope of the license granted herein.
For any further information on product please refer to your local Umicore PMC contact at http://www.pmc.umicore.com
Patented, U.S. Pat. No. 7,250,510. Sold under an exclusive license from Total Synthesis Ltd.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Professor Mike Organ and co-workers have developed the PEPPSI™ (Pyridine-Enhanced Precatalyst Preparation Stabilization and Initiation) precatalysts for palladium-catalyzed cross-coupling reactions.
PEPPSI™ palladium N-heterocyclic-carbene catalyst system enhances efficiency and functional group tolerance in catalysis.
PEPPSI™ palladium N-heterocyclic-carbene catalyst system enhances efficiency and functional group tolerance in catalysis.
PEPPSI™ palladium N-heterocyclic-carbene catalyst system enhances efficiency and functional group tolerance in catalysis.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico





![[Pd(IPr#)(cin)Cl]](/deepweb/assets/sigmaaldrich/product/structures/391/578/9bb7eaef-fa70-4f50-8644-2c55cec3925d/640/9bb7eaef-fa70-4f50-8644-2c55cec3925d.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)




