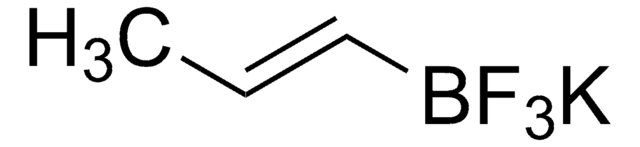
655228
Potassium vinyltrifluoroborate
95%
Sinónimos:
Potassium (ethenyl)trifluoroborate
About This Item
Productos recomendados
assay
95%
form
solid
SMILES string
[K+].F[B-](F)(F)C=C
InChI
1S/C2H3BF3.K/c1-2-3(4,5)6;/h2H,1H2;/q-1;+1
InChI key
ZCUMGICZWDOJEM-UHFFFAOYSA-N
Categorías relacionadas
General description
Potassium vinyltrifluoroborate is an air- and water-stable potassium organotrifluoroborate that can be utilized in coupling reactions under relatively mild conditions.
Application
- Suzuki Miyaura cross-coupling reactions and polymerization reactions
- Synthesis of photonic crystals
- Synthesis of sensitizers for dye-sensitized solar cells
- Mannich / diastereoselective hydroamination reaction sequence
Organotrifluoroborates as versatile and stable boronic acid surrogates.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Potassium trifluoroborates are moisture- and air-stable organoboron reagents suitable for oxidative conditions.
Potassium trifluoroborates are moisture- and air-stable organoboron reagents suitable for oxidative conditions.
Potassium trifluoroborates are moisture- and air-stable organoboron reagents suitable for oxidative conditions.
Potassium trifluoroborates are moisture- and air-stable organoboron reagents suitable for oxidative conditions.
Contenido relacionado
The central theme of the Molander group's research is the development of new synthetic methods and their application to the synthesis of organic molecules.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![[1,1′-bis(difenilfosfino)ferroceno]dicloropaladio(II), complejo con diclorometano](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)



![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)