331570
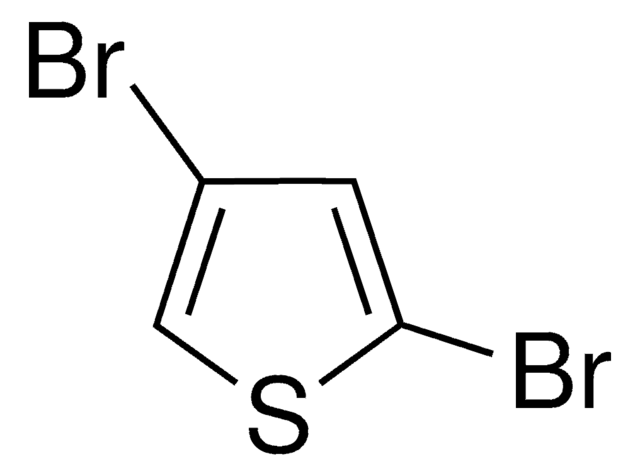
2,5-Diiodothiophene
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C4H2I2S
Número de CAS:
Peso molecular:
335.93
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
98%
form
solid
bp
139-140 °C (lit.)
mp
37-41 °C (lit.)
storage temp.
2-8°C
SMILES string
Ic1ccc(I)s1
InChI
1S/C4H2I2S/c5-3-1-2-4(6)7-3/h1-2H
InChI key
PNYWRAHWEIOAGK-UHFFFAOYSA-N
General description
The multilayer desorption behavior of 2,5-diidothiophene was studied.
Application
2,5-Diiodothiophene was used in the preparation of oligothiophene films. It was used in maskless fabrication of periodic patterns of a conjugated polymer. It was also used in fabrication of oligothiophene and polythiophene micropatterns.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
204.8 °F - closed cup
flash_point_c
96.00 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Sudarshan Natarajan et al.
Langmuir : the ACS journal of surfaces and colloids, 21(15), 7052-7056 (2005-07-13)
Maskless fabrication of periodic patterns of a conjugated polymer is achieved by regioselective condensation of 2,5-diiodothiophene on chemically patterned substrate surfaces followed by in situ photochemical conversion of the condensed molecules into oligothiophenes and polythiophenes. This approach utilizes preferential aggregation
Sudarshan Natarajan et al.
The journal of physical chemistry. B, 110(15), 8047-8051 (2006-04-14)
This paper describes the details of surface reactions producing >100-nm-thick conjugated polymer films. When 2,5-diiodothiophene films deposited on copper are irradiated with UV at room temperature in Ar environments, oligothiophene films are synthesized. The average conjugation length of the produced
Photochemical production of oligothiophene and polythiophene micropatterns from 2, 5-diiodothiophene on Au in UHV.
Liu G, et al.
Surface Science, 592(1), L305-L309 (2005)
Guangming Liu et al.
The journal of physical chemistry. B, 110(41), 20197-20201 (2006-10-13)
The multilayer desorption behavior of 2,5-diidothiophene and the dendritic aggregation of photochemical reaction products during the desorption of 2,5-diiodothiophene multilayers have been studied. Like many other aromatic compounds, 2,5-diiodothiophene shows a multilayer desorption behavior different from the typical zeroth-order kinetics
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico