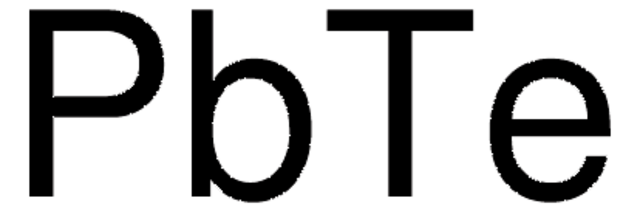
254231
Lead(II) selenide
99.99%
About This Item
Productos recomendados
Quality Level
assay
99.99%
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
density
8.1 g/mL at 25 °C (lit.)
greener alternative category
, Enabling
SMILES string
[Se]=[PbH2]
InChI
1S/Pb.Se
InChI key
GGYFMLJDMAMTAB-UHFFFAOYSA-N
General description
Application
Packaging
signalword
Danger
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 1A - STOT RE 2
Storage Class
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Thermoelectric materials comprise a wide range of solid compounds distinguished by their ability to convert thermal and electrical energy.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico