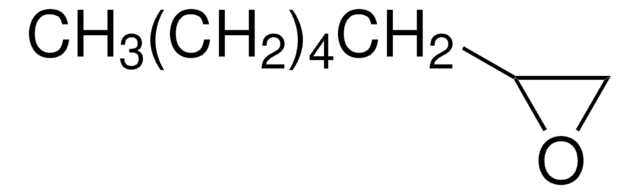C102504
Cyclohexene oxide
98%
Sinónimos:
1,2-Epoxycyclohexane, 7-Oxabicyclo[4.1.0]heptane
About This Item
Productos recomendados
assay
98%
form
liquid
autoignition temp.
703 °F
expl. lim.
12.36 %
refractive index
n20/D 1.452 (lit.)
bp
129-130 °C (lit.)
density
0.97 g/mL at 25 °C (lit.)
SMILES string
C1CCC2OC2C1
InChI
1S/C6H10O/c1-2-4-6-5(3-1)7-6/h5-6H,1-4H2
InChI key
ZWAJLVLEBYIOTI-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- Polymeric carbon nitride with internal np homojunctions for efficient photocatalytic CO2 reduction coupled with cyclohexene oxidation: This study focuses on the use of cyclohexene oxide in the context of photocatalytic CO2 reduction, highlighting the application of polymeric carbon nitride as a catalyst. The process shows how cyclohexene oxide can be efficiently converted in a coupled reaction that also addresses environmental concerns through CO2 reduction (W Zhen et al., 2021).
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 3 - Muta. 2 - Skin Corr. 1B
Storage Class
3 - Flammable liquids
wgk_germany
WGK 1
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![7-Oxabicyclo[4.1.0]heptan-2-one 98%](/deepweb/assets/sigmaaldrich/product/structures/209/639/448778d7-ca19-409d-a52e-8d2866c49812/640/448778d7-ca19-409d-a52e-8d2866c49812.png)













![7-Oxabicyclo[2.2.1]heptane 98%](/deepweb/assets/sigmaaldrich/product/structures/377/935/931d29d9-08c9-492a-b42e-3f8f5a20f595/640/931d29d9-08c9-492a-b42e-3f8f5a20f595.png)