515396
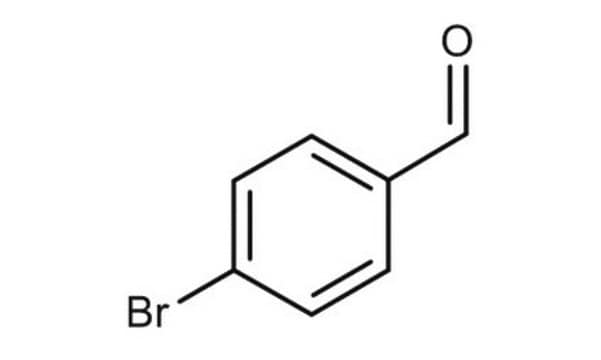
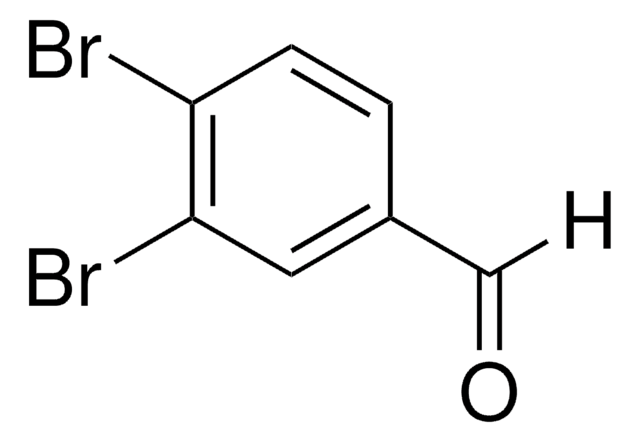
3,5-Dibromobenzaldehyde
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
Br2C6H3CHO
Número de CAS:
Peso molecular:
263.91
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
mp
84-88 °C (lit.)
Quality Level
functional group
aldehyde
bromo
SMILES string
Brc1cc(Br)cc(C=O)c1
InChI
1S/C7H4Br2O/c8-6-1-5(4-10)2-7(9)3-6/h1-4H
InChI key
ZLDMZIXUGCGKMB-UHFFFAOYSA-N
Categorías relacionadas
Application
Reactant involved in:
- Suzuki-Miyaura cross-coupling reactions
- Synthesis of blue fluorescent dye derivatives for organic light emitting diodes
- Sharpless kinetic resolution for the formation of Baylis-Hillman enal adducts
- Synthesis of podophyllotoxin mimetic pyridopyrazoles as anticancer agents
- Allylic alkylation
- Synthesis of C2-symmetric biphosphine ligand I
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Conformational Behavior of Conjugated Polymers With Oligo (phenylene vinylene) Side Chains.
Peeter H and Koeckelberghs G.
Macromolecular Chemistry and Physics, 214(5), 538-546 (2013)
Coordination-Driven Self-Assembly of Fullerene-Functionalized Pt (II) Metallacycles.
Neti VSPK, et al.
Organometallics, 34(20), 4813-4815 (2015)
Synthesis and luminescence characteristics of conjugated dendrimers with 2, 4, 6-triaryl-1, 3, 5-triazine periphery.
Kim CK, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 44(1), 254-263 (2006)
Punit P Seth et al.
Bioorganic & medicinal chemistry letters, 14(22), 5569-5572 (2004-10-16)
The preparation and evaluation of novel aryl urea analogs as broad-spectrum antibacterial agents is described. Numerous compounds showed low micromolar minimum inhibitory concentrations (MIC) against both Gram-positive and Gram-negative bacteria. Selected analogs also exhibited in vivo efficacy in a lethal
Jakub Saadi et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(12), 3842-3845 (2013-02-21)
Double-action haloketones: A super silyl group enabled the first highly diastereoselective Mukaiyama aldol reactions of α-chloro- and α-fluoroketones with a wide range of aldehydes, providing anti-β-siloxy-α-haloketones. This process is compatible with one-pot double-aldol methodology and allows for rapid access to
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico