389439
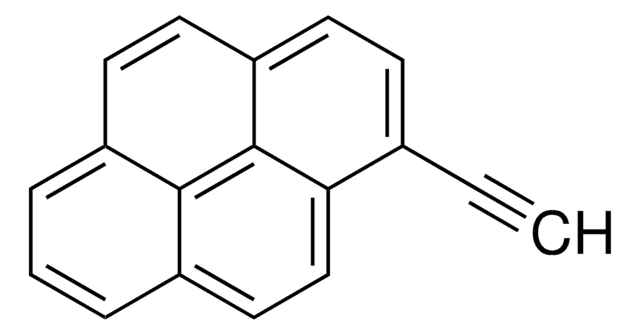
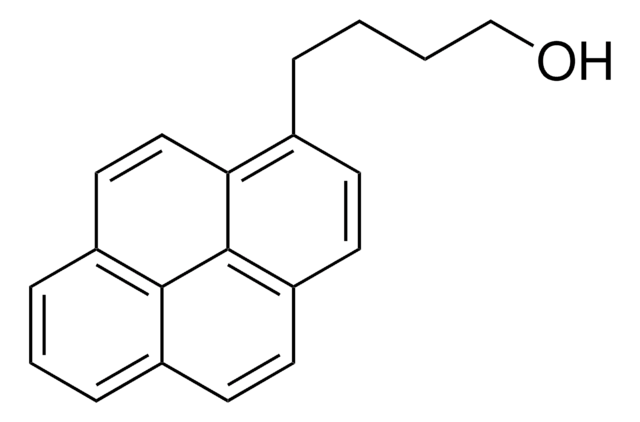
1-Pyrenemethanol
98%
Sinónimos:
1-(1-Hydroxymethyl)pyrene, 1-Hydroxymethylpyrene
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C17H12O
Número de CAS:
Peso molecular:
232.28
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
98%
Formulario
solid
mp
123-126 °C (lit.)
grupo funcional
hydroxyl
cadena SMILES
OCc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C17H12O/c18-10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)17(13)16(11)12/h1-9,18H,10H2
Clave InChI
NGDMLQSGYUCLDC-UHFFFAOYSA-N
Aplicación
1-Pyrenemethanol can be used:
- For the synthesis of pincer-like benzene-bridged fluorescent selective sensor for adenosine-5′-triphosphate (ATP) detection.
- As a starting material for the synthesis of pyrene-end poly(glycidyl methacrylate) polymer.
- As an initiator for the synthesis of pyrene core star polymers.
- For the synthesis of 1-pyrenecarboxaldehyde, an important intermediate in pharmaceutical and agrochemical fields.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Highly efficient oxidation of alcohols to carbonyl compounds in the presence of molecular oxygen using a novel heterogeneous ruthenium catalyst.
Ji H, et al.
Tetrahedron Letters, 43(40), 7179-7183 (2002)
H Glatt et al.
Chemico-biological interactions, 92(1-3), 305-319 (1994-06-01)
1-Hydroxymethylpyrene (HMP) is activated to a potent mutagen, detectable in Salmonella typhimurium, in the presence of hepatic cytosol, cofactor for sulfotransferases, and chloride anions. The number of induced mutations is linear to the amount of cytosol used over a wide
Walter Meinl et al.
Pharmacogenetics, 12(9), 677-689 (2002-12-05)
Various enzymatically formed sulfuric acid esters are chemically reactive and mutagenic. This metabolic activation pathway is not detected in standard in-vitro mutagenicity test systems. We describe the construction of Salmonella typhimurium TA1538-derived strains expressing alloenzymes *1, *2, *3, *5, *6
H Glatt et al.
Chemico-biological interactions, 109(1-3), 195-219 (1998-05-05)
Sulfation is a common final step in the biotransformation of xenobiotics and is traditionally associated with inactivation. However, the sulfate group is electron-withdrawing and may be cleaved off heterolytically in some molecules leading to electrophilic cations which may form adducts
Y J Surh et al.
Carcinogenesis, 11(9), 1451-1460 (1990-09-01)
Our previous studies on 7-hydroxymethyl-12-methylbenz[a]anthracene and 6-hydroxymethylbenzo[a]pyrene showed that cytosolic sulfotransferase activity plays a major role in the formation of hepatic benzylic DNA and RNA adducts by these carcinogens in rats. In the present study, we found similar sulfotransferase activity
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico







![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)