358673
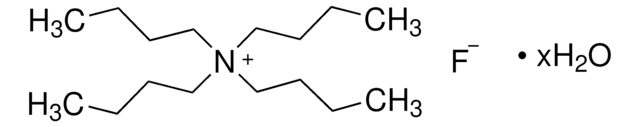
Tetrabutylammonium fluoride on silica gel
~1.5 mmol/g (F-)
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
(CH3CH2CH2CH2)4N(F)
Peso molecular:
261.46
Beilstein:
3570522
Número CE:
Número MDL:
Código UNSPSC:
12352200
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
capacidad
~1.5 mmol/g (F-)
cadena SMILES
[F-].CCCC[N+](CCCC)(CCCC)CCCC
InChI
1S/C16H36N.FH/c1-5-9-13-17(14-10-6-2,15-11-7-3)16-12-8-4;/h5-16H2,1-4H3;1H/q+1;/p-1
Clave InChI
FPGGTKZVZWFYPV-UHFFFAOYSA-M
Categorías relacionadas
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
Riesgos supl.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Mehrdad Balandeh et al.
Journal of the Electrochemical Society, 164(9), G99-G103 (2017-09-12)
Electrochemical fluorination of methyl(phenylthio)acetate was achieved using tetrabutylammonium fluoride (TBAF). Electrochemical fluorination was performed under potentiostatic anodic oxidation using an undivided cell in acetonitrile containing TBAF and triflic acid. The influence of several parameters including: oxidation potential, time, temperature, sonication
Santosh Kumar Behera et al.
Photochemical & photobiological sciences : Official journal of the European Photochemistry Association and the European Society for Photobiology, 14(12), 2225-2237 (2015-10-31)
The mechanism for the dual emission of 2-(4'-N,N-dimethylaminophenyl)imidazo[4,5-c]pyridine (DMAPIP-c) in protic solvents was investigated by synthesizing and studying its analogues. Theoretical calculations were carried out to corroborate the experimental findings. The deprotonation studies suggest that the enhancement in the TICT
Delia López-Velázquez et al.
Carbohydrate polymers, 125, 224-231 (2015-04-11)
We have synthesized and characterized five members of a homologous series of side chain polymers of hydroxypropyl cellulose esters obtained by homogeneous esterification with 6-[4'-(ethoxycarbonyl)biphenyl-4-yloxy]hexanoic acid. Two acylation procedures were studied. One procedure involved the acid chloride derivative and the
Eric P Gillis et al.
Journal of medicinal chemistry, 58(21), 8315-8359 (2015-07-23)
The role of fluorine in drug design and development is expanding rapidly as we learn more about the unique properties associated with this unusual element and how to deploy it with greater sophistication. The judicious introduction of fluorine into a
Soumen Ghosh et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 149, 869-874 (2015-05-24)
A series of Schiff bases synthesized by the condensation of benzohydrazide and -NO2 substituted benzaldehyde have been used as selective fluoride ion sensor. Test paper coated with these synthetic Schiff bases (test kits) can detect fluoride ion selectively with a
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico