UC168
(±)-Bufuralol hydrochloride
≥98% (HPLC), solid, β-Adrenoceptor agonist/antagonist
Sinonimo/i:
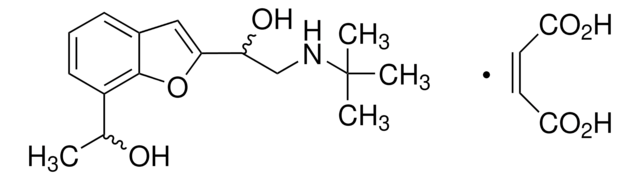
α-[(tert-Butylamino)methyl]-7-ethyl-2-benzofuranmethanol hydrochloride
About This Item
Prodotti consigliati
product name
(±)-Bufuralol hydrochloride,
Forma fisica
solid
Colore
white to off-white
Punto di fusione
143-146 °C
Solubilità
H2O: soluble
methanol: soluble
Temperatura di conservazione
2-8°C
Stringa SMILE
Cl.CCc1cccc2cc(oc12)C(O)CNC(C)(C)C
InChI
1S/C16H23NO2.ClH/c1-5-11-7-6-8-12-9-14(19-15(11)12)13(18)10-17-16(2,3)4;/h6-9,13,17-18H,5,10H2,1-4H3;1H
KJBONRGCLLBWCJ-UHFFFAOYSA-N
Applicazioni
Azioni biochim/fisiol
Caratteristiche e vantaggi
Confezionamento
Nota sulla preparazione
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Discover Bioactive Small Molecules for ADME/Tox
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.