SML2706
Jadomycin B
≥98% (HPLC)
Sinonimo/i:
1-(sec-butyl)-12-((4,5-dihydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-7-hydroxy-5-methyl-8H-benzo[b]oxazolo[3,2-f]phenanthridine-2,8,13(1H,3aH)-trione
About This Item
Prodotti consigliati
Origine biologica
Streptomyces venezuelae
Livello qualitativo
Saggio
≥98% (HPLC)
Stato
powder
Condizioni di stoccaggio
protect from light
Solubilità
DMSO: 1 mg/mL
Temperatura di conservazione
−20°C
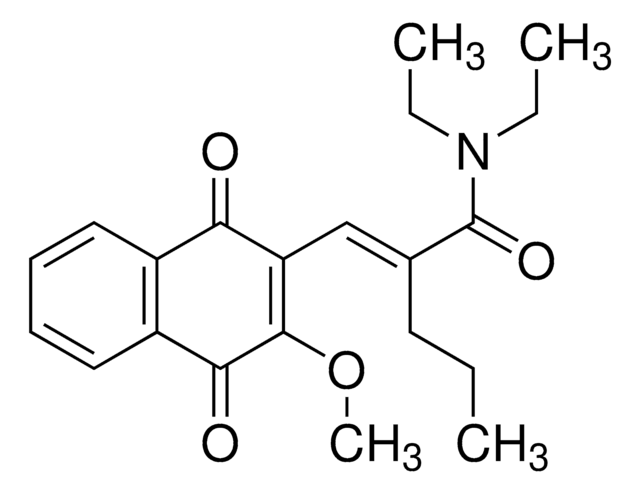
Stringa SMILE
N21C(OC(=O)C2C(CC)C)c3c(c(cc(c3)C)O)C4=C1C(=O)c5c(cccc5OC6OC(C(C(C6)O)O)C)C4=O
InChI
1S/C30H31NO9/c1-5-13(3)24-30(37)40-29-16-9-12(2)10-17(32)21(16)23-25(31(24)29)28(36)22-15(27(23)35)7-6-8-19(22)39-20-11-18(33)26(34)14(4)38-20/h6-10,13-14,18,20,24,26,29,32-34H,5,11H2,1-4H3
BSBSCJRAEMDCHC-UHFFFAOYSA-N
Descrizione generale
Jadomycin B displays antimicrobial, anti-tumor, aurora-B kinase inhibition, DNA cleaving and more activities.3,4,5,6
Jadomycin B was found to be active against a variety of staphylococci, including methicillin-resistant Staphylococcus aureus in a MIC of 1μg/ml.3 In addition, its anti-tumor activity was demonstrated as it kills drug-sensitive and multidrug-resistant breast cancer cell, through inhibition of type II topoisomerases and the induction of DNA damage and apoptosis. Jadomycin B (15 mM), 24-hour treatment significantly lowered the levels of topoisomerase IIa protein versus the vehicle control.4
It was also shown that Jadomycin B inhibits Aurora-B kinase activity by phosphorylation of histone H3 on Ser10 in a dose-dependent manner (10μg /mL Jadomycin B reduced H3 phosphorylation by 70%).5
Jadomycin B was also found to cleave DNA in the presence of Cu (II) by reducing it to Cu(I) which can further react with H2O2 to form hydroxyl radicals that causes DNA strand scission without the requirement of any external reducing agent. The EC50 value of Jadomycin B for single-strand scission was approximately 1.7μM.6
Azioni biochim/fisiol
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.





![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)