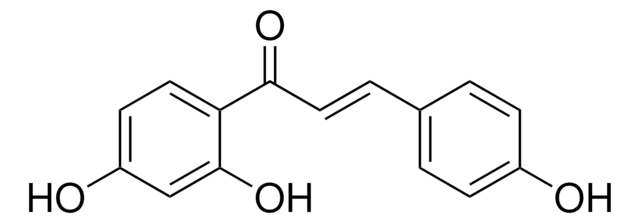
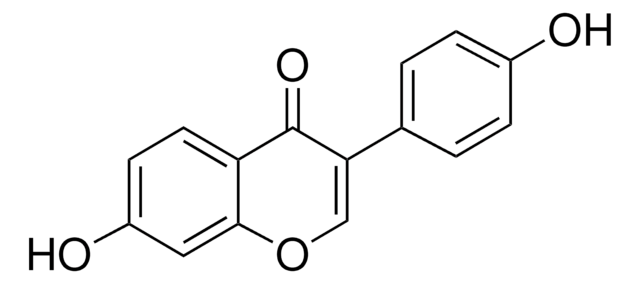
78825
Liquiritigenin
≥97.0% (HPLC)
Sinonimo/i:
7,4′-Dihydroxyflavanone, 7-Hydroxy-2-(4-hydroxyphenyl)-2,3-dihydro-4H-1-benzopyran-4-one
About This Item
Prodotti consigliati
Saggio
≥97.0% (HPLC)
Stato
powder or crystals
Impurezze
≤7% water
applicazioni
metabolomics
vitamins, nutraceuticals, and natural products
Stringa SMILE
Oc1ccc(cc1)[C@@H]2CC(=O)c3ccc(O)cc3O2
InChI
1S/C15H12O4/c16-10-3-1-9(2-4-10)14-8-13(18)12-6-5-11(17)7-15(12)19-14/h1-7,14,16-17H,8H2/t14-/m0/s1
FURUXTVZLHCCNA-AWEZNQCLSA-N
Categorie correlate
Descrizione generale
Applicazioni
- to study its inhibitory effect on tumor metastasis in the treatment of colorectal cancer[2]
- as a reference standard for ultra-performance liquid chromatography (UPLC) of Chaihu-Shugan-San (CSS) extract[3]
- as a potential antiviral drug against hepatitis C virus (HCV) infection[4]
Azioni biochim/fisiol
Confezionamento
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Active Filters
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.