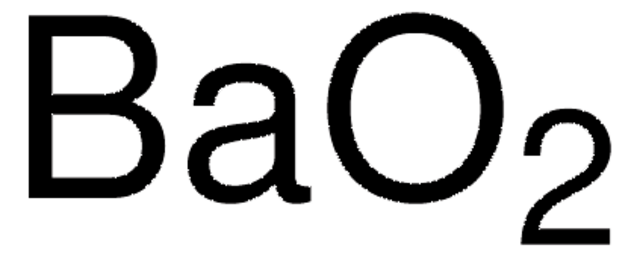208507
Ammonium perchlorate
99.5% trace metals basis
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
NH4ClO4
Numero CAS:
Peso molecolare:
117.49
Numero CE:
Numero MDL:
Codice UNSPSC:
12352300
ID PubChem:
NACRES:
NA.21
Prodotti consigliati
Livello qualitativo
Saggio
99.5% trace metals basis
Forma fisica
powder
Impiego in reazioni chimiche
reagent type: oxidant
Densità
1.95 g/mL at 25 °C (lit.)
Stringa SMILE
N.OCl(=O)(=O)=O
InChI
1S/ClHO4.H3N/c2-1(3,4)5;/h(H,2,3,4,5);1H3
HHEFNVCDPLQQTP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Ammonium perchlorate is widely used as an oxidizing agent. The orthorhombic crystals of NH4ClO4 converts to cubic crystal system at temperatures higher than 240°C. CuO nanorods promotes the thermal degradation of ammonium perchlorate.
Applicazioni
Ammonium perchlorate (NH4ClO4) may be employed for the purification of as-made “pure” mesoporous silicas via oxidation.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Ox. Sol. 1 - STOT RE 2
Codice della classe di stoccaggio
5.1A - Strongly oxidizing hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Mesoporous silicas prepared by ammonium perchlorate oxidation and theirs application in the selective adsorption of high explosives
Yang R, et al.
Microporous and Mesoporous Materials : The Official Journal of the International Zeolite Association, 168, 46-50 (2013)
Synthesis of CuO nanorods and their catalytic activity in the thermal decomposition of ammonium perchlorate
Chen L, et al.
J. Alloy Compounds, 464(1), 532-536 (2008)
Johanna Bünsow et al.
Journal of colloid and interface science, 326(1), 61-65 (2008-08-05)
We report on electrochemically prepared hydrogel layers of poly-N-isopropylacrylamide (pNIPAm) and on the influence that the supporting electrolyte has on their thickness and morphology. Ions that are destabilizing in the Hofmeister sense increase the thickness. The effect correlates well with
Min Zou et al.
Journal of hazardous materials, 225-226, 124-130 (2012-05-29)
Micrometer-sized cobalt oxalates with different morphologies have been prepared in the presence of surfactants. The effect of catalysts morphology on the thermal decomposition of ammonium perchlorate (AP) was evaluated by differential thermal analysis (DSC). Remarkably, contrary to the well-accepted concepts
Mary E Gilbert et al.
Environmental health perspectives, 116(6), 752-760 (2008-06-19)
Perchlorate is an environmental contaminant that blocks iodine uptake into the thyroid gland and reduces thyroid hormones. This action of perchlorate raises significant concern over its effects on brain development. The purpose of this study was to evaluate neurologic function
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.