14340
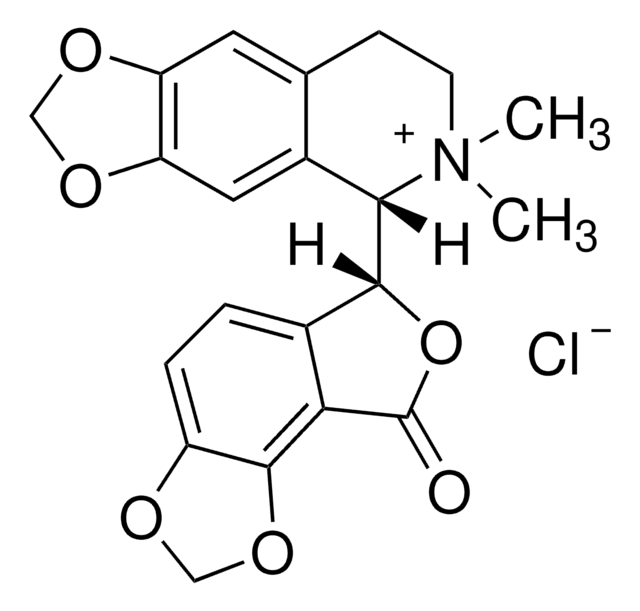
(+)-Bicuculline
≥97.0% (TLC)
Sinonimo/i:
Bucuculline
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥97.0% (TLC)
Forma fisica
powder
Attività ottica
[α]20/D +126±6°, c = 1% in chloroform
Punto di fusione
193-197 °C
Stringa SMILE
[H][C@]1(OC(=O)c2c3OCOc3ccc12)[C@@]4([H])N(C)CCc5cc6OCOc6cc45
InChI
1S/C20H17NO6/c1-21-5-4-10-6-14-15(25-8-24-14)7-12(10)17(21)18-11-2-3-13-19(26-9-23-13)16(11)20(22)27-18/h2-3,6-7,17-18H,4-5,8-9H2,1H3/t17-,18+/m0/s1
IYGYMKDQCDOMRE-ZWKOTPCHSA-N
Informazioni sul gene
rat ... Gabra2(29706)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- as a compound to compare pharmacodynamics and network activity profiles of conolidine/cannabidiol
- to study the effects of chronic caffeine administration on the function of GABAA receptor
- to isolate N-methyl-D-aspartate receptor (NMDAR)-specific evoked and miniature excitatory postsynaptic currents (eEPSCs and mEPSCs) in neurons of rats
Azioni biochim/fisiol
Caratteristiche e vantaggi
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 2 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1
Codice della classe di stoccaggio
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Yeast is one of the most important microorganisms known and utilised by mankind. Ancient Middle Eastern civilisations used the organism to bake bread and to produce mead, beer and wine.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.