02070595
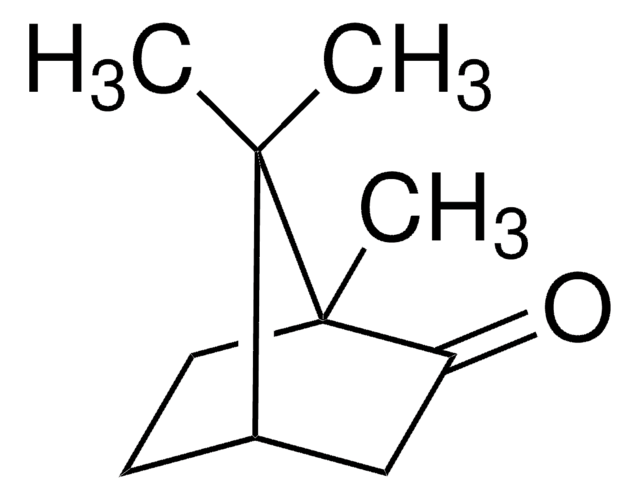
Camphor (dl)
primary reference standard
Sinonimo/i:
(±)-Camphor, 1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one
About This Item
Prodotti consigliati
Grado
primary reference standard
Densità del vapore
5.2 (vs air)
Tensione di vapore
4 mmHg ( 70 °C)
Durata
limited shelf life, expiry date on the label
Limite di esplosione
3.5 %
Produttore/marchio commerciale
HWI
P. eboll.
204 °C (lit.)
Punto di fusione
175-177 °C (lit.)
applicazioni
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
Stringa SMILE
[H][C@](CC1=O)(CC2)C(C)(C)[C@]12C
InChI
1S/C10H16O/c1-9(2)7-4-5-10(9,3)8(11)6-7/h7H,4-6H2,1-3H3/t7-,10+/m1/s1
DSSYKIVIOFKYAU-XCBNKYQSSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Exact content by quantitative NMR can be found on the certificate.
Applicazioni
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Inhalation - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 2 Inhalation
Codice della classe di stoccaggio
4.1B - Flammable solid hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
147.9 °F - closed cup
Punto d’infiammabilità (°C)
64.4 °C - closed cup
Choose from one of the most recent versions:
Certificati d'analisi (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.