8.02954
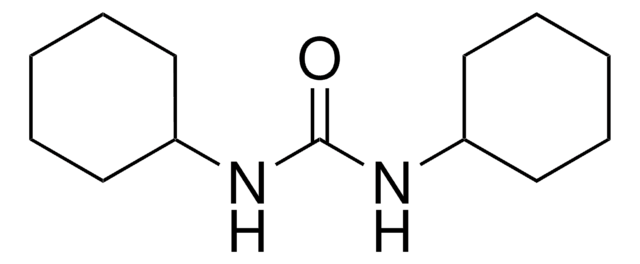
N,N′-Dicyclohexylcarbodiimide
for peptide synthesis
Sinonimo/i:
N,N′-Dicyclohexylcarbodiimide, DCC
About This Item
Prodotti consigliati
product name
N,N′-Dicyclohexylcarbodiimide, for synthesis
Livello qualitativo
Forma fisica
solid
Potenza
1110 mg/kg LD50, oral (Rat)
71 mg/kg LD50, skin (Rat)
Impiego in reazioni chimiche
reaction type: Coupling Reactions
P. eboll.
148-152 °C/15 hPa
Punto di fusione
35-36 °C
Temp. transizione
flash point 113 °C
Densità
0.95 g/cm3 at 40 °C
Densità bulk
920 kg/m3
applicazioni
peptide synthesis
Temperatura di conservazione
2-30°C
InChI
1S/C13H22N2/c1-3-7-12(8-4-1)14-11-15-13-9-5-2-6-10-13/h12-13H,1-10H2
QOSSAOTZNIDXMA-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Applicazioni
- The synthesis of optically pure N-acyl-N,N′-dicyclohexylureas.
- The activation of the carboxylic acid groups in aromatic carboxylic acids to facilitates their reaction with (N-isocyanimino)trifluoroacetamide to form the corresponding 1,3,4-oxadiazole derivatives.
- The synthesis of poly (vinyl alcohol-co-vinyl levulinate) copolymers for use in biomedical applications.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Sens. 1
Codice della classe di stoccaggio
6.1D - Non-combustible, acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
235.4 °F - closed cup
Punto d’infiammabilità (°C)
113 °C - closed cup
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.