T30805
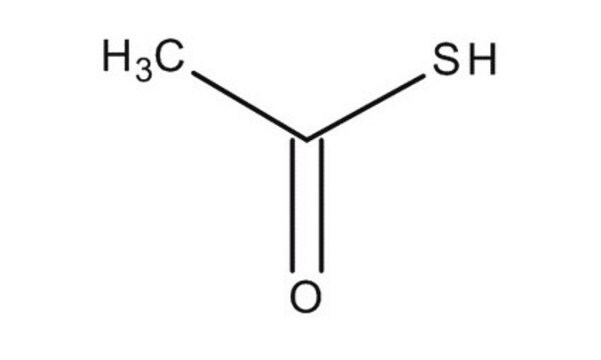
Thioacetic acid
96%
Sinonimo/i:
TAA, TMA, Thiacetic acid
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
96%
Indice di rifrazione
n20/D 1.465 (lit.)
P. eboll.
88-91.5 °C (lit.)
Densità
1.065 g/mL at 25 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
CC(S)=O
InChI
1S/C2H4OS/c1-2(3)4/h1H3,(H,3,4)
DUYAAUVXQSMXQP-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- Enantioselective addition to nitroalkenes to form chiral 1,2-aminothiol derivatives in the presence of a novel sulfinyl urea organocatalyst. This method has been successfully employed in the synthesis of antifungal drug, sulconazole.
- Asymmetric Michael addition reaction with chalcones in the presence of a bifunctional amine thiourea catalyst to form synthetically useful thioesters.
- Asymmetric 1,6-conjugate addition with para-quinone methides in the presence of a chiral phosphoric acid catalyst to form chiral sulfur-containing diphenylmethane-type compounds.
- Conjugate addition to methacrylamides with chiral trans-2,5-disubstituted pyrrolidine auxiliaries to form chiral β-mercaptocarboxylic acid derivatives.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Eye Dam. 1 - Flam. Liq. 2 - Skin Sens. 1
Codice della classe di stoccaggio
3 - Flammable liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
64.4 °F - closed cup
Punto d’infiammabilità (°C)
18 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.