S5006
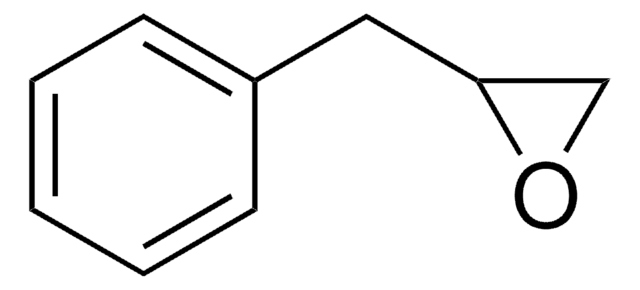
Styrene oxide
97%
Sinonimo/i:
1,2-Epoxyethylbenzene, Phenylethylene oxide, Phenyloxirane
About This Item
Prodotti consigliati
Densità del vapore
4.14 (vs air)
Tensione di vapore
<1 mmHg ( 20 °C)
Saggio
97%
Temp. autoaccensione
928 °F
Limite di esplosione
~22 %
Indice di rifrazione
n20/D 1.535 (lit.)
P. eboll.
194 °C (lit.)
Punto di fusione
−37 °C (lit.)
Densità
1.054 g/mL at 25 °C (lit.)
Stringa SMILE
C1OC1c2ccccc2
InChI
1S/C8H8O/c1-2-4-7(5-3-1)8-6-9-8/h1-5,8H,6H2
AWMVMTVKBNGEAK-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
- Synthesis of poly (styrene oxide) with different molecular weights using tin catalysts: This study explores the ring-opening polymerization of styrene oxide using tin catalysts to produce homopolymers with varying molecular weights (Kayan, 2015).
- Electrogenerated BF3 from tetrafluoroborate-based ionic liquids: theoretical and experimental studies towards selective styrene oxide isomerization: Research on using electrogenerated BF3 to selectively isomerize styrene oxide, highlighting theoretical and experimental insights (Bortolami et al., 2021).
- Selective conversion of styrene oxide to 2-phenylethanol in cascade reactions over non-noble metal catalysts: This paper investigates the catalytic conversion of styrene oxide to 2-phenylethanol using non-noble metal catalysts (Sasu et al., 2016).
- Laboratory blueprints for interstellar searches of aromatic chiral molecules: rotational signatures of styrene oxide: Study of the rotational spectra of styrene oxide for potential detection in interstellar space (Stahl et al., 2020).
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Carc. 1B - Eye Irrit. 2 - Muta. 1B - Skin Irrit. 2 - Skin Sens. 1
Codice della classe di stoccaggio
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
176.0 °F - closed cup
Punto d’infiammabilità (°C)
80 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.