900624
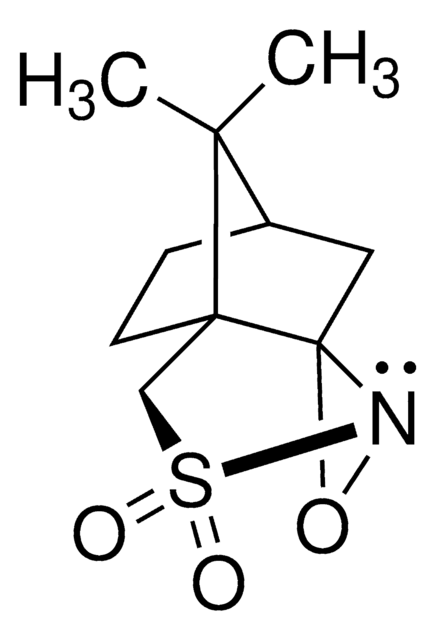
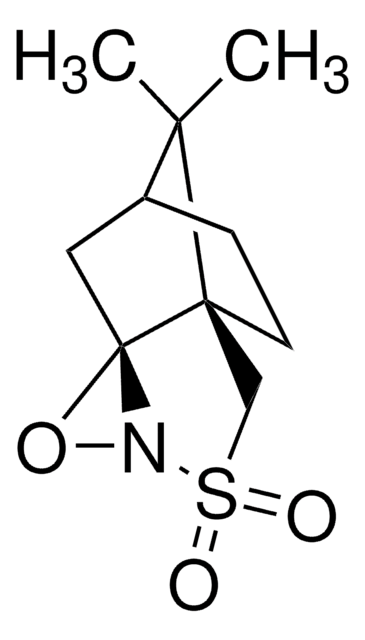
Di-t-butyl oxaziridine
≥95%
Sinonimo/i:
Kurti oxaziridine
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥95%
Forma fisica
liquid
Disponibilità
available only in USA
Indice di rifrazione
n/D 1.4453
Densità
0.90 g/mL
Temperatura di conservazione
2-8°C
Stringa SMILE
CC(C)(C)C1(NO1)C(C)(C)C
Applicazioni
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Self-react. C
Codice della classe di stoccaggio
5.2 - Organic peroxides and self-reacting hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
143.6 °F
Punto d’infiammabilità (°C)
62 °C
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Contenuto correlato
Amines and their derivatives are ubiquitous substances since they make up the overwhelming majority of drug molecules, agrochemicals as well as many compounds that are produced by plants and living organisms (i.e., natural products).
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.