803448
SDAD (NHS-SS-Diazirine) (succinimidyl 2-[(4,4′-azipentanamido)ethyl]-1,3′-dithiopropionate)
About This Item
Prodotti consigliati
Saggio
≥90%
Forma fisica
powder
PM
388.46
Impiego in reazioni chimiche
reagent type: cross-linking reagent
Condizioni di stoccaggio
desiccated
Solubilità
DMSO or DMF: soluble
Condizioni di spedizione
ambient
Temperatura di conservazione
2-8°C
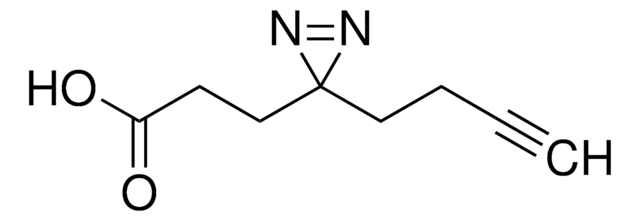
Stringa SMILE
CC1(N=N1)CCC(NCCSSCCC(ON2C(CCC2=O)=O)=O)=O
InChI
1S/C14H20N4O5S2/c1-14(16-17-14)6-4-10(19)15-7-9-25-24-8-5-13(22)23-18-11(20)2-3-12(18)21/h2-9H2,1H3,(H,15,19)
NLPWBELUEANJAT-UHFFFAOYSA-N
Descrizione generale
Caratteristiche e vantaggi
- Membrane-permeable—suitable for in vivo intracellular protein crosslinking
- Heterobifunctional—NHS ester group reacts with primary amines at pH 7 to 9 to form covalent amide bonds; diazirine (azipentanoate) group reacts efficiently with any amino acid side chain or peptide backbone upon activation with long-wave UV light (330-370 nm)
- Controllable—two-step chemical crosslinking is activated using common laboratory UV lamps
- Easy to use—these crosslinkers are photo-stable under typical laboratory lighting conditions so there is no need to perform experiments in the dark
- Better than aryl azides—the diazirine photoreactive group has better photostability in normal light than phenyl azide groups of traditional photoreactive crosslinkers, yet the diazirine group is more efficiently activated by long-wave UV light
Avvertenza
Prodotti correlati
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.