494224
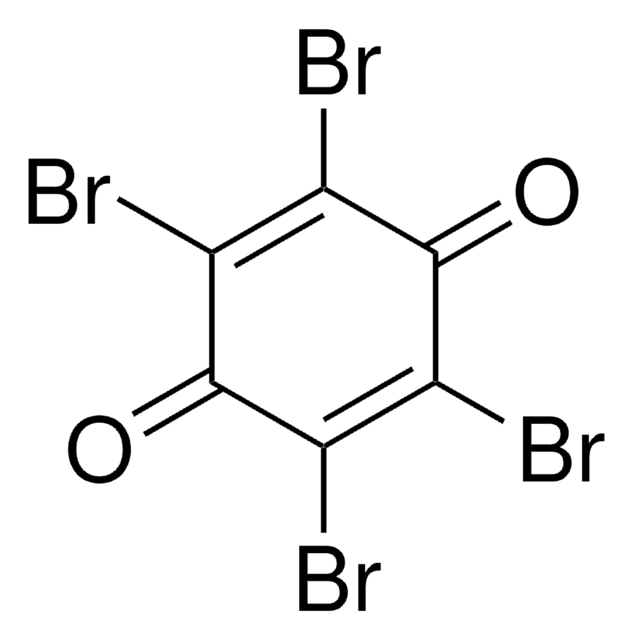
Tetrabromohydroquinone
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6Br4-1,4-(OH)2
Numero CAS:
Peso molecolare:
425.69
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
Prodotti consigliati
Saggio
98%
Punto di fusione
252-255 °C (lit.)
Stringa SMILE
Oc1c(Br)c(Br)c(O)c(Br)c1Br
InChI
1S/C6H2Br4O2/c7-1-2(8)6(12)4(10)3(9)5(1)11/h11-12H
DTFQULSULHRJOA-UHFFFAOYSA-N
Descrizione generale
Tetrabromohydroquinone (TBHQ), also known as 2,3,5,6-tetrabromohydroquinone, can be synthesized by reacting bromanil with phenyl phosphine. It crystallizes in the monoclinic space group, P21/n. The effect of C-Br group on the crystal structure of TBHQ has been investigated. Its role in causing green luminescence in Ptychodera flava has been investigated.
Applicazioni
Tetrabromohydroquinone may be used in the synthesis of thermoresponsive branched oligoethylene glycol dendrimers.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Mona A Abdel-Rahman et al.
European journal of medicinal chemistry, 69, 848-854 (2013-10-15)
Three interesting thermoresponsive branched oligoethylene glycol dendrimers based on tetrabromohydroquinone were efficiently synthesized from tetrabromohydroquinone and three different oligoethylene glycol derivatives. By visual inspection, all these dendrimers are water-soluble at room temperature. The thermoresponsive behaviors were investigated by using UV/vis
Akira Kanakubo et al.
Luminescence : the journal of biological and chemical luminescence, 20(6), 397-400 (2005-06-21)
2,3,5,6-Tetrabromohydroquinone was isolated as a luminous substance from Ptychodera flava. This compound emitted light after addition of hydrogen peroxide under basic conditions. Since hydroquinone had no fluorescence, further investigation by spectral analysis revealed that riboflavin was the only possible light
Reaction of phenylphosphine with p-quinones.
Pudovik AN, et al.
Russian Chemical Bulletin, 27(7), 1450-1452 (1978)
A comparative study of the crystal structures of tetrahalogenated hydroquinones and ?-hydroquinone.
Thalladi VR, et al.
Acta Crystallographica Section B, Structural Science, Crystal Engineering and Materials, 55(6), 1005-1013 (1999)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.

![4,7-Dibromobenzo[c][1,2,5]thiadiazole 95%](/deepweb/assets/sigmaaldrich/product/structures/711/964/3fd3ffd1-5916-468e-a743-22f1611b5a33/640/3fd3ffd1-5916-468e-a743-22f1611b5a33.png)




