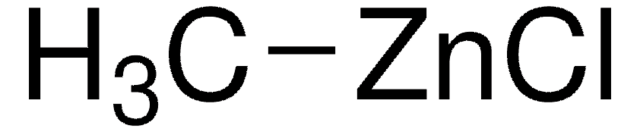417246
Dimethylzinc solution
1.0 M in heptane
Sinonimo/i:
Dimethylzinc
About This Item
Prodotti consigliati
Forma fisica
liquid
Concentrazione
1.0 M in heptane
P. eboll.
44-46 °C
Densità
0.724 g/mL at 25 °C
Stringa SMILE
C[Zn]C
InChI
1S/2CH3.Zn/h2*1H3;
AXAZMDOAUQTMOW-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Dimethylzinc is a diorganozinc reagent and nucleophile used in the synthesis of propargylic amines.
Applicazioni
- A catalyst with nickel for the stereoselective C−2 alkenylation and dialkenylation of pyridine derivatives by alkynes to give monoalkenylation products.
- A reagent with aldehydes and 2-methoxyaniline for the synthesis of enantioselective alkyl and aralkyl secondary amines via one-pot three-component coupling reaction in the presence of zirconium tetraisopropoxide.
- A methylating reagent for methylation of fluoroalkylated pyruvates in the presence of copper/chiral diphosphine catalyst.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Aquatic Acute 1 - Aquatic Chronic 1 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 2
Organi bersaglio
Central nervous system
Codice della classe di stoccaggio
4.2 - Pyrophoric and self-heating hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
30.2 °F - closed cup
Punto d’infiammabilità (°C)
-1 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.