176974
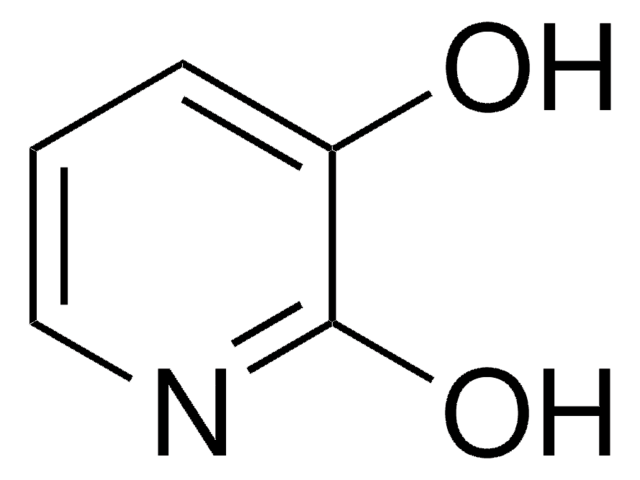
2,4-Dihydroxypyridine
97%
Sinonimo/i:
2,4-Pyridinediol, 3-Deazauracil, 4-Hydroxy-2-pyridone
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C5H5NO2
Numero CAS:
Peso molecolare:
111.10
Beilstein:
108533
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
Prodotti consigliati
Saggio
97%
Stato
solid
Punto di fusione
272-276 °C (lit.)
Stringa SMILE
Oc1ccnc(O)c1
InChI
1S/C5H5NO2/c7-4-1-2-6-5(8)3-4/h1-3H,(H2,6,7,8)
ZEZJPIDPVXJEME-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
2,4-Dihydroxypyridine (3-deazauracil) is a potent inhibitor of dihydrouracil dehydrogenase.
Applicazioni
2,4-Dihydroxypyridine (3-deazauracil) was used in the synthesis of diazaphenoxathiin skeleton.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
J Molgó et al.
Journal de pharmacologie, 16 Suppl 2, 109-144 (1985-01-01)
In this review the effects of aminopyridines and chemically related compounds are documented in an attempt to analyse the mechanism underlying their presynaptic actions at the vertebrate neuromuscular junction. Aminopyridines and related compounds are of particular interest because they greatly
K T Lin et al.
Therapeutic drug monitoring, 5(4), 491-496 (1983-01-01)
A rapid and simple procedure for liquid chromatographic analysis of plasma 3-deazauridine (3-DU), an antineoplastic agent, was developed. The plasma was extracted with methanolic silver acetate to remove interfering ultraviolet-absorbing materials and the 3-DU partially purified on a small anion
Maria Teresa Cocco et al.
European journal of medicinal chemistry, 38(1), 37-47 (2003-02-21)
Bis(pyridyl)methane derivatives 5-40 were obtained from the reaction of 4-hydroxy-2-pyridones 3 and 4 with aldehydes. Compounds 5-40 were evaluated for cytotoxic activity against a panel of 60 human cancer cell lines by the National Cancer Institute and some of them
M T Cocco et al.
European journal of medicinal chemistry, 35(5), 545-552 (2000-07-12)
4-hydroxy-2-pyridone derivatives 2 were prepared by reaction of 3-amino-3-dialkylaminopropenoates with bis(2,4, 6-trichlorophenyl)malonate. These compounds were further reacted with a set of aldehydes to give bis(pyridyl)methanes 3 and 4. The newly synthesized compounds 2, 3 and 4 were evaluated in vitro
F N Naguib et al.
Biochemical pharmacology, 38(9), 1471-1480 (1989-05-01)
One hundred and five nucleobase analogues were screened as inhibitors of dihydrouracil dehydrogenase (DHUDase, EC 1.3.1.2) from mouse liver. 5-Benzyloxybenzyluracil, 1-deazauracil (2,6-pyridinediol), 3-deazauracil (2,4-pyridinediol), 5-benzyluracil, 5-nitrobarbituric acid and 5,6-dioxyuracil (alloxan) were identified as potent inhibitors of this activity, with apparent
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.