15404
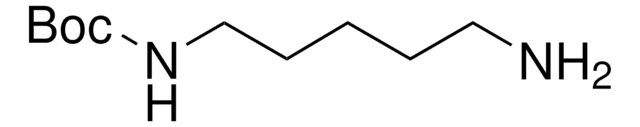
N-Boc-1,4-butanediamine
≥97.0% (GC/NT)
Sinonimo/i:
N-Boc-1,4-diaminobutane, tert-Butyl N-(4-aminobutyl)carbamate
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥97.0% (GC/NT)
Impiego in reazioni chimiche
reagent type: cross-linking reagent
Indice di rifrazione
n20/D 1.460
Densità
0.984 g/mL at 20 °C (lit.)
Gruppo funzionale
Boc
amine
Stringa SMILE
NCCCCNC(OC(C)(C)C)=O
InChI
1S/C9H20N2O2/c1-9(2,3)13-8(12)11-7-5-4-6-10/h4-7,10H2,1-3H3,(H,11,12)
ZFQWJXFJJZUVPI-UHFFFAOYSA-N
Categorie correlate
Applicazioni
- Carboxy-Silane Coated Iron Oxide Nanoparticles: Details the application of N-Boc-1,4-butanediamine in modifying iron oxide nanoparticles for imaging and drug delivery (D Stanicki, S Boutry, S Laurent, et al., 2014). Access the article.
Altre note
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Skin Corr. 1B
Codice della classe di stoccaggio
8A - Combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
228.2 °F - closed cup
Punto d’infiammabilità (°C)
109.0 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Mono-Boc-protected diamines are versatile building blocks for chemical synthesis. Their production is a lot more challenging than the simple reaction scheme might imply, because the Boc-anhydride reagent cannot differentiate between the two identical amino moieties in the substrate.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.