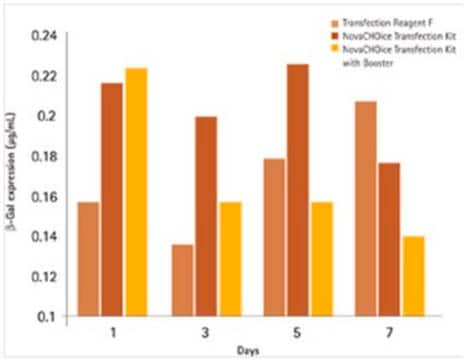
Transfection efficiency is affected by many different things, including plasmid size and purity, media components present, transfection reagent selected, amount of DNA and transfection reagent used, cell density, etc. Optimizing the protocol with respect to these concerns will allow you to achieve a higher transfection efficiency. For many cell lines and transfection reagents, optimized protocols are already available.
NPT01
NeuroPorter™ Transfection Kit
Lipid formulation for nucleic acid transfections in neuronal and glial cells
Synonyme(s) :
NeuroPorter Transfection, Transfection Kit
Sélectionner une taille de conditionnement
744.00 CHF
Date d'expédition estimée le18 mars 2025
Sélectionner une taille de conditionnement
About This Item
744.00 CHF
Date d'expédition estimée le18 mars 2025
Produits recommandés
Qualité
for molecular biology
Niveau de qualité
Forme
dried film
Utilisation
kit sufficient for 75-200 transfections
Disponibilité
available only in USA, Canada and EU
Technique(s)
transfection: suitable
Température de stockage
2-8°C
Description générale
Application
- C6 glioma (human)
- Cortical neurons (rat primary)
- Dorsal Root Ganglion (DRG) cells (rat)
- NT2 neurons(human precursor cells)
- NT neurons (human differentiated cells)
- Subventricular Zone (SVZ) cells (mouse)
- White matter cells (mouse)
Caractéristiques et avantages
- Optimized for primary neurons, glial cells, and cultured neural cell lines
- Very low toxicity with no neuro-degeneration or dendrite withdrawal
- Efficient DNA delivery primary neurons, glial cells, and cultured neural cell lines
- Fast and easy to use compared to other methods
- Compatible with both serum and serum-free transfection protocols
Composants
1.5 mL Hydration Buffer H9036
7.5 mL DNA Diluent D1941
Attention
Principe
Informations légales
Produit(s) apparenté(s)
Code de la classe de stockage
12 - Non Combustible Liquids
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Transfection introduces genetic material into cells, aiding research in gene expression and cell biology.
This brief webinar provides an overview of what transfection is and the methods that are used to introduce DNA or RNA into eukaryotic cells.
Contenu apparenté
Browse our convenient transfection reagent selection guide to match the best reagent for your specific cell line and application needs.
-
How can I increase the efficiency of my transfection?
1 answer-
Helpful?
-
-
What is the difference between stable and transient transfection?
1 answer-
When the DNA enters the nucleus of the cell, the plasmid is replicated by the cell machinery (transient transfection). During this time, RNA is transcribed and protein translated until the plasmid DNA is lost after a few cell divisions. This expression of the plasmid DNA, mRNA, and protein is transient (temporary).In some cases, the plasmid DNA is integrated into the host cell genome. This is usually accompanied by forced expression using a selection antibiotic and sometimes a cloning step (to be sure all cells have the same integration site). Once the DNA is stable, the cell line can be frozen and used to express protein for many years. Clones may even be screened for those expressing the highest amount of protein.
Helpful?
-
-
What quality does the DNA need to be in order to use it for transfection?
1 answer-
The DNA needs to be good quality or it may cause the cells to lyse and/or they won't transfect efficiently. Plasmid DNA prepared with a column-based DNA purification kit is suitable for transfections. Sigma's GenElute™ Minprep, Midiprep and Maxiprep kits work well for DNA plasmid purification. After preparing the DNA, confirm the OD A260:A280 ratio is greater than 1.6 for use in plasmid transfections.
Helpful?
-
-
Why do I see a precipitate in my cell culture after lipid-based transfection?
1 answer-
The precipitate is likely excess lipid or EDTA and will probablly not affect transfection efficiency. If your DNA plasmid is suspended in TE, be sure the concentration of EDTA is <0.3 mM, or suspend the DNA in sterile molecular biology grade water instead.
Helpful?
-
-
Why are neurons difficult to transfect?
1 answer-
Neurons are both non-dividing cells, and sensitive to toxicity from the transfection reagent used. Sigma's Neuroporter™ Transfection Reagent is an optimized transfection reagent with a ready-to-use protocol that is helping overcome these limitations.
Helpful?
-
-
Is optimizing the transfection protocol important?
1 answer-
For many common cell lines, transfection reagent efficiency is very high and the protocols will not require any optimization. For hard-to-transfect cells or those ultimately expressing a toxic protein, the protocol should be optimized for best transfection efficiency. Taking time to optimize will give you more transfected cells with each procedure, which can mean more protein expressed and results that are reproducible.
Helpful?
-
-
Can antibiotics be present in the medium during transfection?
1 answer-
We recommend that no antibiotics are present during transfection. The process of transfection can make the cells somewhat more porous to allow for efficient DNA entry. During this time, antibiotics will also enter the cells more easily and the cells may show increased cell death. Wait until about 24 hours after transfection to resume the use of preventative antibiotics and/or start the use of selective antibiotics.
Helpful?
-
-
What is transfection efficiency?
1 answer-
Transfection efficiency is a measure of how many cells take up the DNA during the transfection process. Many transfection reagents can achieve a transfection efficiency of >90% in common cell lines. Other cell lines are hard to transfect, and require special reagents and/or techniques to achieve even a small population of transfected cells.
Helpful?
-
-
Is low cell passage number an important consideration for transfection?
1 answer-
Yes, we recommend cells are at a low passage when being used for any application, including transfection. The reason why depends on what type of cells they are. Primary cells will undergo a finite number of divisions, and as they get closer to senesence they divide more slowly - both affecting their ability to take up DNA (transient transfection), and minimizing their abillity to incorporate the DNA into the genome (stable selection).Cultured common cell lines are often immortalized, and generally continue to aquire mutations, leading to a heterogenous population that may perform differently from cells of lower passage number - leading to results that are not reproducible.
Helpful?
-
-
How do I choose a transfection reagent?
1 answer-
There are many guides that help you select a transfection reagent. In general, consider:The type of cell(s) you will transfectThe type of nucleic acid or protein you will introduce to the cellThe composition of your cell culture mediumThe need for stable or transient transfectionThe equipment you have availableThe other factors important to you - cost, protocol flexibility, ease of use, etc.
Helpful?
-
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique