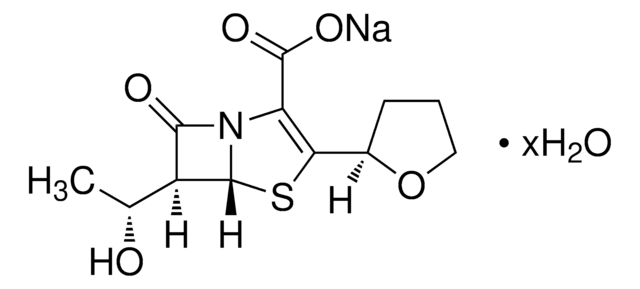
Cefepime hydrochloride is soluble in several solvents. It is highly soluble in water and normal saline and it can also be dissolved in dextrose solutions. It is also easily soluble in methanol and diethylether and slightly soluble in ethanol, but concentrations will need to be determined empirically in all cases, as solubility is not tested internally.
PHR1763
Cefepime hydrochloride
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(e):
Cefepime hydrochloride, Cefepime dihydrochloride monohydrate
Größe auswählen
Größe auswählen
About This Item
Empfohlene Produkte
Qualität
certified reference material
pharmaceutical secondary standard
Qualitätsniveau
Agentur
traceable to Ph. Eur. Y0000633
traceable to USP 1097636
API-Familie
cefepime
Analysenzertifikat (CofA)
current certificate can be downloaded
Verpackung
pkg of 1 g
Methode(n)
HPLC: suitable
gas chromatography (GC): suitable
Anwendung(en)
pharmaceutical (small molecule)
Format
neat
Lagertemp.
2-8°C
SMILES String
[s]1[c]([nH]c(c1)\C(=N\OC)\C(=O)N[C@H]2[C@H]3SCC(=C(N3C2=O)C(=O)[O-])C[N+]4(CCCC4)C)=N.Cl.Cl.O
InChI
1S/C19H24N6O5S2.2ClH.H2O/c1-25(5-3-4-6-25)7-10-8-31-17-13(16(27)24(17)14(10)18(28)29)22-15(26)12(23-30-2)11-9-32-19(20)21-11;;;/h9,13,17H,3-8H2,1-2H3,(H3-,20,21,22,26,28,29);2*1H;1H2/b23-12-;;;/t13-,17-;;;/m1.../s1
InChIKey
LRAJHPGSGBRUJN-OMIVUECESA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Pharmaceutical secondary standard for application in quality control. Provides pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Anwendung
Hinweis zur Analyse
Sonstige Hinweise
Fußnote
Ähnliches Produkt
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Dokumente section.
Wenn Sie Hilfe benötigen, wenden Sie sich bitte an Kundensupport
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
-
I'd like to know hos I can solubilize thie product (Cefepime PHR1763) and what is the solubility limit. Thanks in advance.
1 answer-
Helpful?
-
-
May I know what is the recommended media for reconstitution? I would also like to know if there is any information pertaining to the estimated shelf life for both the neat and the working solution.
1 answer-
PHR1763, Cefepime hydrochloride is a Pharmaceutical Secondary Standard, intended for use in compendial testing. The preparation of the standard is per the relevant compendial monograph. The shelf life for the solid neat product is per the expiration date on the accompanying COA - a sample COA can be found in the document section of the product webpage (linked below). Shelf life of the prepared solutions is per the applicable monograph or general chapter.
https://www.sigmaaldrich.com/CA/en/product/sial/phr1763#product-documentationHelpful?
-
Active Filters
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.