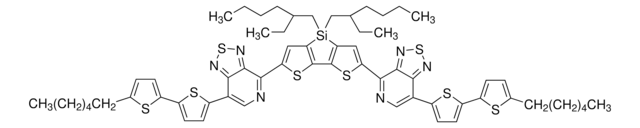
901058
J51
Synonym(e):
Poly[(5,6-difluoro-2-octyl-2H-benzotriazole-4,7-diyl)-2,5-thiophenediyl[4,8-bis[5-(2-hexyldecyl)-2-thienyl]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl]-2,5-thiophenediyl]
About This Item
Empfohlene Produkte
Beschreibung
Band gap: 1.99 eV
Qualitätsniveau
Form
solid
Mol-Gew.
Mw 40,000-80,000 by GPC (PS standard)
Grünere Alternativprodukt-Eigenschaften
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp (Schmelzpunkt)
>200 °C
Löslichkeit
organic solvents: soluble (Soluble in chlorobenzene and dichlorobenzene, Limited solubility in CHCl3)
Energie der Orbitale
HOMO -5.29 eV
LUMO -3.3 eV
PDI
2.0‑3.0
Grünere Alternativprodukt-Kategorie
Allgemeine Beschreibung
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Artikel
Novel biosensing devices, like bio-FET sensors, offer label-free analysis by detecting ionic and biomolecular charges, crucial for diagnostics and biological research.
Professor Chen (Nankai University, China) and his team explain the strategies behind their recent record-breaking organic solar cells, reaching a power conversion efficiency of 17.3%.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.


![Poly-[(9,9-di-n-octylfluorenyl-2,7-diyl)-alt-(benzo[2,1,3]thiadiazol-4,8-diyl)] average Mn ≤25000](/deepweb/assets/sigmaaldrich/product/structures/428/661/1c4ebb98-9d51-48c0-96c7-e556ca425aa4/640/1c4ebb98-9d51-48c0-96c7-e556ca425aa4.png)