04473
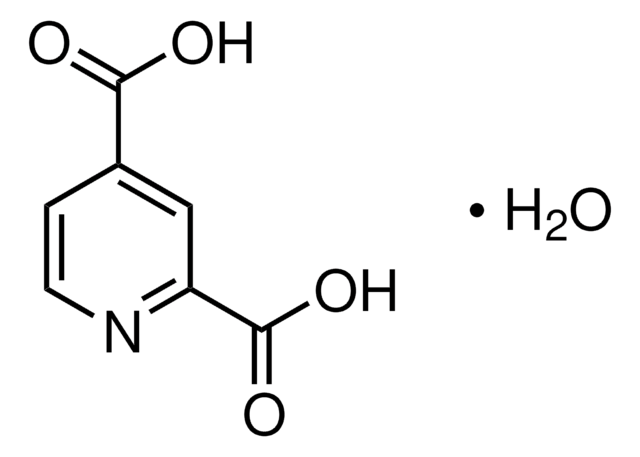
2,4-Pyridinedicarboxylic acid
≥98.0%
Synonyme(s) :
Lutidinic acid
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C7H5NO4
Numéro CAS:
Poids moléculaire :
167.12
Numéro Beilstein :
131631
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352106
ID de substance PubChem :
Nomenclature NACRES :
NA.25
Produits recommandés
Niveau de qualité
Pureté
≥98.0%
98.0-102.0% (T)
Chaîne SMILES
OC(=O)c1ccnc(c1)C(O)=O
InChI
1S/C7H5NO4/c9-6(10)4-1-2-8-5(3-4)7(11)12/h1-3H,(H,9,10)(H,11,12)
Clé InChI
MJIVRKPEXXHNJT-UHFFFAOYSA-N
Application
2,4-Pyridinedicarboxylic acid is an in vitro and in cell inhibitor, as well as a known inhibitor of the histone lysine demethylases. 2,4-Pyridinedicarboxylic acid has been used in a study to determine that ruthenium(II) complexes exert antimetastatic effects on several tumor cell lines in vitro, achieved mostly by the effect on cell adhesion, migration and angiogenesis. . 2,4-Pyridinedicarboxylic acid has been used in a study to develop an assay that represents the first report of a RapidFire mass spectrometery assay for an epigenetics target.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Gunnar Schley et al.
The American journal of pathology, 181(5), 1595-1606 (2012-09-05)
The role of proximal versus distal tubular injury in the pathogenesis of acute kidney injury (AKI) is debatable. Inhibition of prolyl hydroxylases that regulate the degradation of hypoxia-inducible transcription factors (HIFs) is a promising therapeutic approach to optimize energy preservation
Nevenka Gligorijević et al.
Journal of inorganic biochemistry, 108, 53-61 (2012-01-24)
In our previous study, ruthenium(II)-p-cymene complexes of general formula [(η(6)-p-cymene)Ru(L)Cl2], L: 3-acetylpyridine (1), 2-amino-5-chloropyridine (2); and [(η(6)-p-cymene)Ru(HL)Cl], HL: 2,3-pyridinedicarboxylic acid (3), 2,4-pyridinedicarboxylic acid (4), revealed low antiproliferative activity, except complex [(η(6)-p-cymene)RuCl(picolinic acid)]·H(2)O (5) which exhibited IC(50) around 80 μM. In
H M Hanauske-Abel
Journal of hepatology, 13 Suppl 3, S8-15 (1991-01-01)
The hydrophilic compound pyridine 2,4-dicarboxylate (2,4-PDCA), designed as a mechanism-based competitive inhibitor of prolyl 4-hydroxylase, is efficiently excluded by the cytoplasmic membrane, but permeates the endoplasmic membrane via a 2,4-PDCA-selective translocator to reach its target enzyme in the intracisternal space.
G Tschank et al.
The Biochemical journal, 275 ( Pt 2), 469-476 (1991-04-15)
The biochemical and morphological consequences of procollagen prolyl 4-hydroxylase inhibition by pyridine-2,4-dicarboxylic acid (2,4-PDCA) and its diethyl ester (diethyl-2,4-PDC) were studied in chick-embryo calvaria, which predominantly synthesize type I collagen. Half-maximal inhibition of tissue hydroxyproline formation required 650 microM-2,4-PDCA, whereas
Line H Kristensen et al.
The FEBS journal, 279(11), 1905-1914 (2012-03-17)
Dynamic methylations and demethylations of histone lysine residues are important for gene regulation and are facilitated by histone methyltransferases and histone demethylases (HDMs). KDM5B/Jarid1B/PLU1 is an H3K4me3/me2-specific lysine demethylase belonging to the JmjC domain-containing family of histone demethylases (JHDMs). Several
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique