C80105
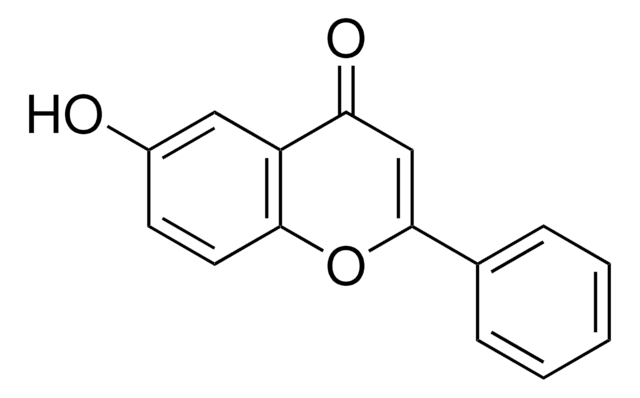
Chrysin
≥96.5% purity, powder
Synonyme(s) :
5,7-Dihydroxyflavone
About This Item
Produits recommandés
product name
Chrysin, ≥96.5%
Niveau de qualité
Pureté
≥96.5%
Forme
powder
Couleur
beige
Pf
284-286 °C (lit.)
Solubilité
0.1 M NaOH: 0.008 g/L
λmax
348 nm
Application(s)
diagnostic assay manufacturing
hematology
histology
Température de stockage
room temp
Chaîne SMILES
Oc1cc(O)c2C(=O)C=C(Oc2c1)c3ccccc3
InChI
1S/C15H10O4/c16-10-6-11(17)15-12(18)8-13(19-14(15)7-10)9-4-2-1-3-5-9/h1-8,16-17H
Clé InChI
RTIXKCRFFJGDFG-UHFFFAOYSA-N
Informations sur le gène
human ... CDC2(983) , CDK5(1020) , CDK6(1021) , CYP19A1(1588) , CYP1A2(1544) , GSK3A(2931)
mouse ... Hexa(15211) , Ptgs2(19225)
rat ... Gabra2(29706)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Chrysin has been used to study the effect of bioflavonoids on the inhibition of ATP synthase in Escherichia coli.
- It has been used to study the effect of Chrysin on eIF4E (eukaryotic translation initiation factor 4E) expression in AGS cell (human gastric carcinoma cell) line.
- It has been used to study the effect of chrysin on human ovarian cancer cells.
Actions biochimiques/physiologiques
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique