704415
Vinylboronic acid MIDA ester
97%
Synonyme(s) :
6-Methyl-2-vinyl-1,3,6,2-dioxazaborocane-4,8-dione
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill) :
C7H10BNO4
Numéro CAS:
Poids moléculaire :
182.97
Numéro MDL:
Code UNSPSC :
12352103
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Essai
97%
Forme
powder
Pf
152-156 °C
Température de stockage
2-8°C
Chaîne SMILES
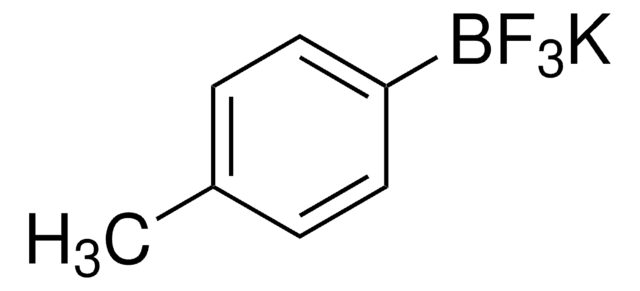
CN1CC(=O)OB(OC(=O)C1)C=C
InChI
1S/C7H10BNO4/c1-3-8-12-6(10)4-9(2)5-7(11)13-8/h3H,1,4-5H2,2H3
Clé InChI
MGRQGYAVASCCAK-UHFFFAOYSA-N
Catégories apparentées
Description générale
Vinylboronic acid MIDA ester, like other MIDA boronates, possesses the capacity for controlled, in situ slow-release of boronic acids under aqueous basic conditions allowing the cross-coupling of classically challenging substrates.
Application
MIDA boronates as stable boronic acid surrogates for classically challenging cross-couplings
Suzuki Cross-Coupling with MIDA Boronates
Suzuki Cross-Coupling with MIDA Boronates
- Vinylboronic acid MIDA ester is an air and chromatographically stable boronic acid surrogate for Suzuki-Miyaura cross-coupling. It can also be used in Heck and oxidative Heck reactions as well as in olefin metathesis to provide the cross-coupled product.
- It is compatible with a wide range of common synthetic reagents that allows functionalization to synthesize structurally complex boronic acid surrogates.
- It undergoes cyclopropanation and epoxidation to yield corresponding MIDA cyclopropane and oxirane, respectively.
- It can be used as one of the major reagents for the scalable synthesis of potent cytotoxin, Leiodermatolide and for the total synthesis of (−)-Blepharocalyxin D.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
A general solution for unstable boronic acids: slow-release cross-coupling from air-stable MIDA boronates.
Knapp DM, et al.
Journal of the American Chemical Society, 131(20), 6961-6963 (2009)
Total Synthesis of (−)-Blepharocalyxin D and Analogues.
Cons BD, et al.
Organic Letters, 15(8), 2046-2049 (2013)
(1-Bromovinyl)-MIDA boronate: a readily accessible and highly versatile building block for small molecule synthesis.
Woerly EM, et al.
Tetrahedron, 69(36), 7732-7740 (2013)
Synthesis of trans-2-(Trifluoromethyl) cyclopropanes via Suzuki reactions with an N-methyliminodiacetic acid boronate.
Duncton MA and Singh R.
Organic Letters, 15(17), 4284-4287 (2013)
Vinyl MIDA boronate: a readily accessible and highly versatile building block for small molecule synthesis.
Uno BE, et al.
Tetrahedron, 65(16), 3130-3138 (2009)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique




![[1,1′-bis(diphénylphosphino)ferrocène]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)