112992
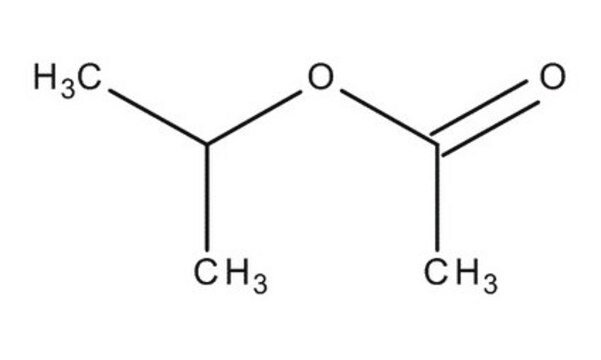
Isopropyl acetate
98%
Synonyme(s) :
Acetic acid isopropyl ester
About This Item
Produits recommandés
Densité de vapeur
3.5 (vs air)
Pression de vapeur
47 mmHg ( 20 °C)
Pureté
98%
Forme
liquid
Température d'inflammation spontanée
894 °F
Limite d'explosivité
1.8 %, 37 °F
8 %
Caractéristiques du produit alternatif plus écologique
Safer Solvents and Auxiliaries
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Indice de réfraction
n20/D 1.377 (lit.)
Point d'ébullition
85-91 °C (lit.)
Pf
−73 °C (lit.)
Densité
0.872 g/mL at 25 °C (lit.)
Autre catégorie plus écologique
, Aligned
Chaîne SMILES
CC(C)OC(C)=O
InChI
1S/C5H10O2/c1-4(2)7-5(3)6/h4H,1-3H3
Clé InChI
JMMWKPVZQRWMSS-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Flam. Liq. 2 - STOT SE 3
Organes cibles
Central nervous system
Risques supp
Code de la classe de stockage
3 - Flammable liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
41.0 °F - closed cup
Point d'éclair (°C)
5 °C - closed cup
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Contenu apparenté
Pourquoi devrait-on choisir entre solvants écologiques et solvants fiables ? Nous vous proposons les deux à la fois grâce à nos solutions biorenouvelables et plus écologiques. Le solvant Cyrene™ est une nouvelle alternative aprotique dipolaire aux solvants courants frappés par la restriction REACH, tels que la N-méthyl-2-pyrrolidone (NMP) et le diméthylformamide (DMF).
Why should you have to choose between solvents that are ecological and those that are reliable? Enjoy both at once with our biorenewable and greener solutions. Cyrene™ solvent is a new dipolar aprotic alternative to common REACH restricted solvents, such as N methyl-2-pyrrolidone (NMP) and Dimethylformamide (DMF).
Why should you have to choose between solvents that are ecological and those that are reliable? Enjoy both at once with our biorenewable and greener solutions. Cyrene™ solvent is a new dipolar aprotic alternative to common REACH restricted solvents, such as N methyl-2-pyrrolidone (NMP) and Dimethylformamide (DMF).
Why should you have to choose between solvents that are ecological and those that are reliable? Enjoy both at once with our biorenewable and greener solutions. Cyrene™ solvent is a new dipolar aprotic alternative to common REACH restricted solvents, such as N methyl-2-pyrrolidone (NMP) and Dimethylformamide (DMF).
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique