ALD00578
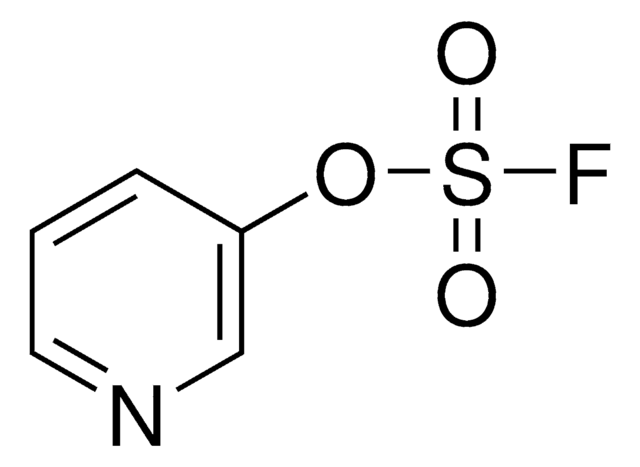
2-Bromopyridin-3-yl sulfurofluoridate
95%
About This Item
Produits recommandés
Niveau de qualité
Pureté
95%
Forme
liquid
Indice de réfraction
n20/D 1.5160
Température de transition
flash point >230 °F
Densité
1.8283 g/mL
Température de stockage
2-8°C
Catégories apparentées
Application
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Skin Corr. 1B
Code de la classe de stockage
8A - Combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Articles
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
This article details the latest progress in synthesizing alkylsulfonyl fluorides through different approaches that utilize photoredox catalysis, electrocatalysis, transition-metal catalysis, and organocatalysis. The alkylsulfonyl fluorides thus prepared could be utilized further in sulfur(VI) fluoride exchange (SuFEx) click reactions.
Contenu apparenté
The Sharpless Lab pursues useful new reactivity and general methods for selectively controlling chemical reactions.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique