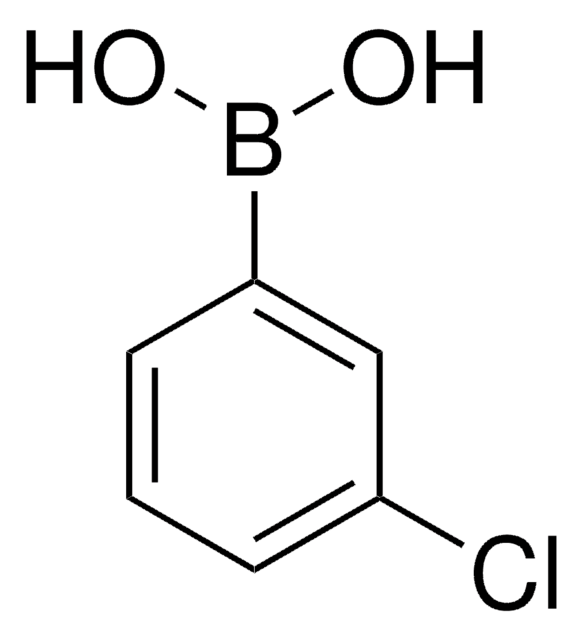
B75956
4-Bromophenylboronic acid
≥95.0%
Synonyme(s) :
(p-Bromophenyl)boronic acid, 4-Bromobenzeneboronic acid, 4-Bromophenylboric acid, p-Bromobenzeneboronic acid, p-Bromophenylboric acid, NSC 25407
About This Item
Produits recommandés
Pureté
≥95.0%
95%
Forme
crystals
Pf
284-288 °C (lit.)
Chaîne SMILES
OB(O)c1ccc(Br)cc1
InChI
1S/C6H6BBrO2/c8-6-3-1-5(2-4-6)7(9)10/h1-4,9-10H
Clé InChI
QBLFZIBJXUQVRF-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- Palladium catalyzed Suzuki-Miyaura cross-couplings
- Pd(II)-catalyzed diastereoselective conjugate additions
- Palladium-catalyzed stereoselective Heck-type reaction of allylic esters with arylboronic acids
- Tandem-type Pd(II)-catalyzed oxidative Heck reaction and intramolecular C-H amidation sequence
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides
- Pd-catalyzed arylative cyclization of alkyne-tethered enals or enones via carbopalladation of alkynes
- Copper-catalyzed cross-couplings
Reagent used in Preparation of
- Gallate-based obovatol analogs with potential anti-tumor activity
- Protein modulators and enzymatic and kinase inhibitors
Autres remarques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique

![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)