M72609
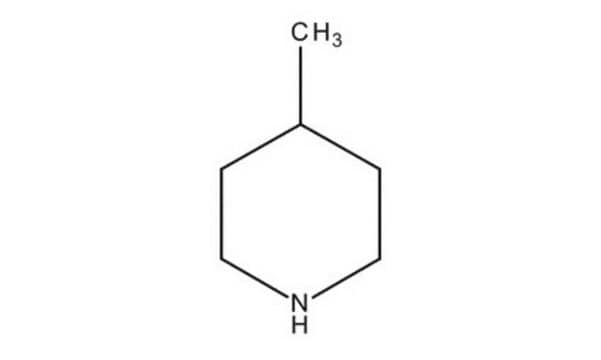
N-Methylpiperidine
99%
Synonym(s):
1-Methylpiperidine
About This Item
Recommended Products
Assay
99%
refractive index
n20/D 1.4378 (lit.)
bp
106-107 °C (lit.)
density
0.816 g/mL at 25 °C (lit.)
SMILES string
CN1CCCCC1
InChI
1S/C6H13N/c1-7-5-3-2-4-6-7/h2-6H2,1H3
InChI key
PAMIQIKDUOTOBW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- sp3 C-H Bond activation with ruthenium(II) catalysts and C(3)-alkylation of cyclic amines
- One-pot synthesis of Z-cinnamic acids
Reactant for synthesis of:
- Unsymmetrical ureas
- Antibacterial imidazolium, pyrrolidinium, and piperidinium salts
- C1-C16 segment of goniodomin A via palladium-catalyzed organostannane thioester coupling
- Multi-targeted inhibitors of insulin-like growth factor-1 receptor and members of ErbB-family receptor kinases
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
37.4 °F - closed cup
Flash Point(C)
3 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
FAS, or 'forever chemicals,' persist in the environment and pose risks to human health. Discover our environmental monitoring tools for PFAS quantification, aiding researchers, regulators, and labs in testing for PFAS.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service