69110
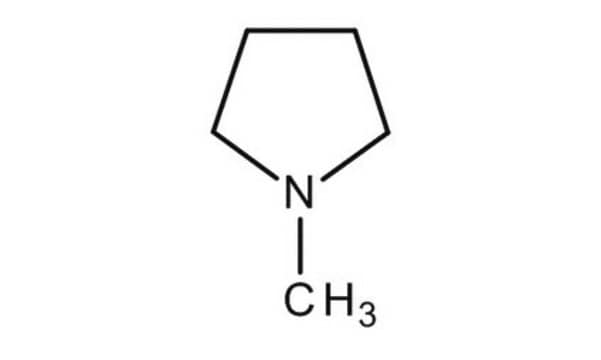
1-Methylpyrrolidine
≥98.0% (GC)
Synonym(s):
N-Methylpyrrolidine, N-Methyltetrahydropyrrole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H11N
CAS Number:
Molecular Weight:
85.15
Beilstein:
102445
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (GC)
form
liquid
refractive index
n20/D 1.425
bp
76-80 °C (lit.)
density
0.800 g/mL at 20 °C (lit.)
SMILES string
CN1CCCC1
InChI
1S/C5H11N/c1-6-4-2-3-5-6/h2-5H2,1H3
InChI key
AVFZOVWCLRSYKC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1-Methylpyrrolidine is extensively used in the synthesis of pyrrolidine based ionic liquids. Some of the other reported applications include:
- Synthesis of ionic liquid electrolytes for primary Li/CFx batteries.
- Preparation of ionic liquid as a reaction medium to carry out sulfuric acid-catalyzed conversion of alkynes to ketones.
- MCM-47, a highly crystalline ferrierite layered silicate, can be synthesized using a diquaternary ammonium salt prepared from the reaction of 1-methylpyrrolidine with 1,4-dibromobutane.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Aquatic Chronic 2 - Flam. Liq. 2 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-0.4 °F - closed cup
Flash Point(C)
-18 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
MCM-47: a highly crystalline silicate composed of hydrogen-bonded ferrierite layers.
Burton A, et al.
Chemistry of Materials, 12(10), 2936-2942 (2000)
Sulfuric acid-catalyzed conversion of alkynes to ketones in an ionic liquid medium under mild reaction conditions.
Wong W L, et al.
ACS Catalysis, 1(2), 116-119 (2011)
N-Methyl-N-propylpiperidinium bis (trifluoromethanesulfonyl) imide (PP13?TFSI)?novel electrolyte base for Li battery.
Sakaebe H and Matsumoto H
Electrochemical Communications, 5(7), 594-598 (2003)
Structure and properties of high stability geminal dicationic ionic liquids.
Anderson J L, et al.
Journal of the American Chemical Society, 127(2), 593-604 (2005)
Ionic liquid electrolytes for lithium batteries: Synthesis, electrochemical, and cytotoxicity studies.
Madria N, et al.
Journal of Power Sources, 234, 277-284 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service