Y0000726
Dorzolamide hydrochloride
European Pharmacopoeia (EP) Reference Standard
About This Item
Produtos recomendados
grau
pharmaceutical primary standard
família API
dorzolamide
fabricante/nome comercial
EDQM
aplicação(ões)
pharmaceutical (small molecule)
formato
neat
cadeia de caracteres SMILES
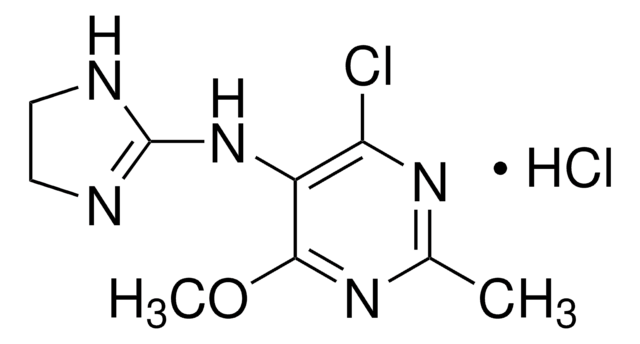
Cl.CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O
InChI
1S/C10H16N2O4S3.ClH/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16;/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16);1H/t6-,8-;/m0./s1
chave InChI
OSRUSFPMRGDLAG-QMGYSKNISA-N
Informações sobre genes
human ... CA2(760)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Embalagem
Outras notas
produto relacionado
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - STOT RE 2
Órgãos-alvo
Central nervous system,Gastrointestinal tract,Bone,Blood,Bladder
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Choose from one of the most recent versions:
Certificados de análise (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica