Y0000529
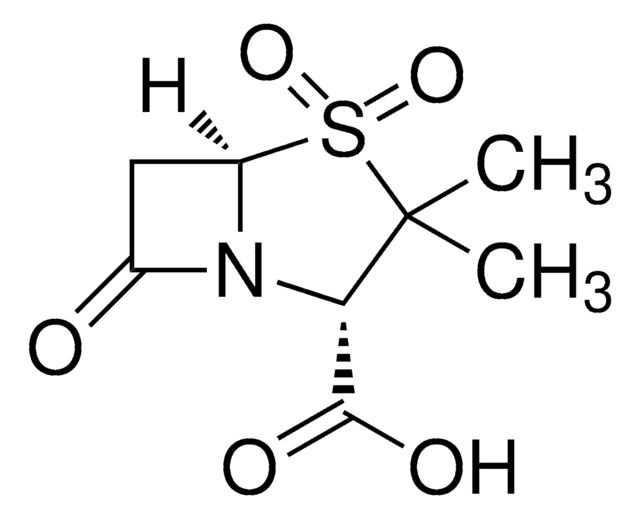
Sulbactam sodium
European Pharmacopoeia (EP) Reference Standard
Sinônimo(s):
Sulbactam sodium salt
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Produtos recomendados
grau
pharmaceutical primary standard
família API
sulbactam
fabricante/nome comercial
EDQM
aplicação(ões)
pharmaceutical (small molecule)
formato
neat
temperatura de armazenamento
2-8°C
InChI
1S/C8H11NO5S.Na/c1-8(2)6(7(11)12)9-4(10)3-5(9)15(8,13)14;/h5-6H,3H2,1-2H3,(H,11,12);/q;+1/p-1/t5-,6+;/m1./s1
chave InChI
NKZMPZCWBSWAOX-IBTYICNHSA-M
Categorias relacionadas
Descrição geral
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Aplicação
Sulbactam sodium EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Embalagem
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Outras notas
Sales restrictions may apply.
produto relacionado
Nº do produto
Descrição
Preços
Choose from one of the most recent versions:
Certificados de análise (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Rui Li et al.
Journal of molecular modeling, 19(6), 2519-2524 (2013-03-05)
The imine intermediates of tazobactam and sulbactam bound to SHV-1 β-lactamase were investigated by molecular dynamics (MD) simulation respectively. Hydrogen bond networks around active site were found different between tazobactam and sulbactam acyl-enzymes. In tazobactam imine intermediate, it was observed
In vitro susceptibilities of clinical isolates of Escherichia coli and Klebsiella species to CSE1034 and other β-lactams.
Manu Chaudhary et al.
The Journal of antibiotics, 66(8), 495-497 (2013-04-25)
Y-T Lee et al.
European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology, 32(9), 1211-1220 (2013-04-05)
Tigecycline (TG) has been shown to be active in vitro against Acinetobacter baumannii, although data on the clinical efficacy of TG alone or in combination for the treatment of infections due to multidrug-resistant A. baumannii (MDRAB) remain limited. The purpose
Alaa A Hassan et al.
Archiv der Pharmazie, 346(7), 562-570 (2013-06-19)
(E)-4-Aryl-2-[2-(1-substituted ethylidene)hydrazinyl]thiazoles and (Z)-3-substituted-4-aryl-2-[(E)-(1-phenylethylidene)hydrazono]-2,3-dihydrothiazoles were synthesized by the reaction of (substituted ethylidene)hydrazinecarbothioamides with ω-bromoacetophenones. The characterization of this new class of compounds was performed using different spectroscopic tools. The structure of (Z)-3-benzyl-4-(4-bromophenyl)-2-[(E)-(1-phenylethylidene)hydrazono]-2,3-dihydrothiazole 6e was unambiguously confirmed by single-crystal X-ray crystallography.
Maura S de Oliveira et al.
Clinics (Sao Paulo, Brazil), 68(4), 569-573 (2013-06-20)
The objective of this study was to evaluate whether the outcomes of carbapenem-resistant Acinetobacter infections treated with ampicillin/sulbactam were associated with the in vitro susceptibility profiles. Twenty-two infections were treated with ampicillin/sulbactam. The median treatment duration was 14 days (range:
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica