8.55019
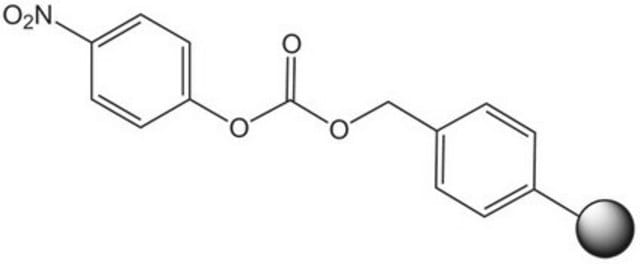
p-Nitrophenyl carbonate Wang resin
Novabiochem®
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Código UNSPSC:
12352005
Produtos recomendados
Nível de qualidade
linha de produto
Novabiochem®
forma
beads
adequação da reação
reaction type: Fmoc solid-phase peptide synthesis
reactivity: amine reactive
fabricante/nome comercial
Novabiochem®
aplicação(ões)
peptide synthesis
temperatura de armazenamento
15-25°C
Categorias relacionadas
Descrição geral
Resin-bound p-nitrophenyl carbonate esters react readily with amines to provide the corresponding resin-bound carbamate. Leznoff [1] was the first to demonstrate the utility of such resins in solid phase synthesis with the preparation of mono-acylated diamines from symmetrical diamines. Similarly, Dressman, et al. [2] have used carbamate linked resin-bound amino acid amides to make hydantoins via a base-mediated cyclization/cleavage strategy. Amines linked to p-nitrophenyl carbonate Wang resin possess similar chemical properties to methoxybenzyloxycarbonyl protected amines. The resin-bound carbamate is, therefore, cleaved with TFA or hydrogenolysis to afford the free amine [3]. Alternatively, cleavage by reduction with lithium aluminium hydride can be used to generate N-methylamines [4], and Raju & Kogan [5] have utilized this resin to prepare sulfonamides by acylation of immobilized arylamines and cleavage with LiOH or NaOMe. An analogous p-nitrophenyl carbonate resin prepared from NovaSyn TGA resin was employed as a support for the solid phase immobilization of amidines [6]. This resin was also used to synthesize polyamines [7, 8], and to immobilize indoles [9] and guanidinylating reagents [10]. Loading of this resin can be quantified by measuring the release of p-nitrophenolate [11].
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] D. M. Dixit, et al. (1978) Israel J. Chem., 17, 248.
[2] B. A. Dressman, et al. (1996) Tetrahedron Lett., 37,937.
[3] J. R. Hauske, et al. (1995) Tetrahedron Lett., 36, 1589.
[4] C. Y. Ho & M. J. Kukla (1997) Tetrahedron Lett., 38, 2799.
[5] B. Raju & T. P. Kogan (1997) Tetrahedron Lett., 38, 3373.
[5] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[6] R. Mohan, et al. (1998) Bioorg. Med. Chem. Lett., 8, 1877.
[7] S. Tomasi, et al. (1998) Bioorg. Med. Chem. Lett., 8, 635.
[8] N. D. Hone & L. J. Payne (2000) Tetrahedron Lett., 41, 6149.
[9] A. L. Smith, et al. (2000) Bioorg. Med. Chem. Lett., 10, 2693.
[10] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[11] A. Paio, et al. (2003) Tetrahedron Lett., 44, 1867.
Associated Protocols and Technical Articles
Protocols for Loading of Peptide Synthesis Resins
Literature references
[1] D. M. Dixit, et al. (1978) Israel J. Chem., 17, 248.
[2] B. A. Dressman, et al. (1996) Tetrahedron Lett., 37,937.
[3] J. R. Hauske, et al. (1995) Tetrahedron Lett., 36, 1589.
[4] C. Y. Ho & M. J. Kukla (1997) Tetrahedron Lett., 38, 2799.
[5] B. Raju & T. P. Kogan (1997) Tetrahedron Lett., 38, 3373.
[5] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[6] R. Mohan, et al. (1998) Bioorg. Med. Chem. Lett., 8, 1877.
[7] S. Tomasi, et al. (1998) Bioorg. Med. Chem. Lett., 8, 635.
[8] N. D. Hone & L. J. Payne (2000) Tetrahedron Lett., 41, 6149.
[9] A. L. Smith, et al. (2000) Bioorg. Med. Chem. Lett., 10, 2693.
[10] A. K. Ghosh, et al. (2001) J. Org. Chem., 66, 2161.
[11] A. Paio, et al. (2003) Tetrahedron Lett., 44, 1867.
Ligação
Replaces: 01-64-0123
Nota de análise
Color (visual): white to yellow to beige
Appearance of substance (visual): beads
Loading / photometric determination of p-nitrophenol released upon treatment with piperidine / DMF: 0.60 - 1.20 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB ), 100 - 200 mesh.
Appearance of substance (visual): beads
Loading / photometric determination of p-nitrophenol released upon treatment with piperidine / DMF: 0.60 - 1.20 mmol/g
Swelling Volume (in DMF): lot specific result
The polymer matrix is copoly (styrene - 1% DVB ), 100 - 200 mesh.
Informações legais
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica