420201
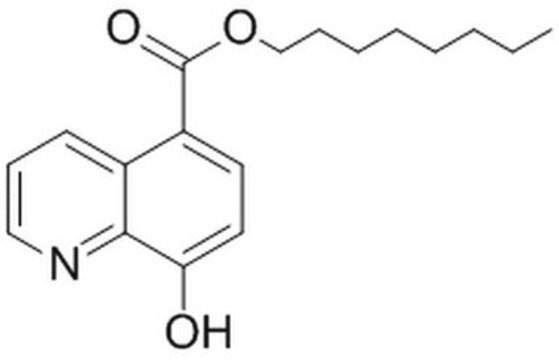
JMJD2 Inhibitor, 5-carboxy-8HQ
The JMJD2 Inhibitor, 5-carboxy-8HQ, also referenced under CAS 5852-78-8, controls the biological activity of JMJD2. This small molecule/inhibitor is primarily used for Phosphorylation & Dephosphorylation applications.
Sinônimo(s):
JMJD2 Inhibitor, 5-carboxy-8HQ, JHDM Inhibitor I, Histone Lysine Demethylase Inhibitor II, 5-carboxy-8HQ, IOX1
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥95% (HPLC)
forma
solid
fabricante/nome comercial
Calbiochem®
condição de armazenamento
OK to freeze
protect from light
cor
orange
solubilidade
DMSO: 50 mg/mL
Condições de expedição
ambient
temperatura de armazenamento
2-8°C
InChI
1S/C10H7NO3/c12-8-4-3-7(10(13)14)6-2-1-5-11-9(6)8/h1-5,12H,(H,13,14)
chave InChI
JGRPKOGHYBAVMW-UHFFFAOYSA-N
Descrição geral
This probe is supplied in conjunction with the Structural Genomics Consortium (SGC). For further characterization details, please visit the IOX1 probe summary on the SGC website.
Embalagem
Advertência
Reconstituição
Outras notas
King, D.N.F., et al. 2010. PLoS One5, e15535.
Informações legais
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica