G10806
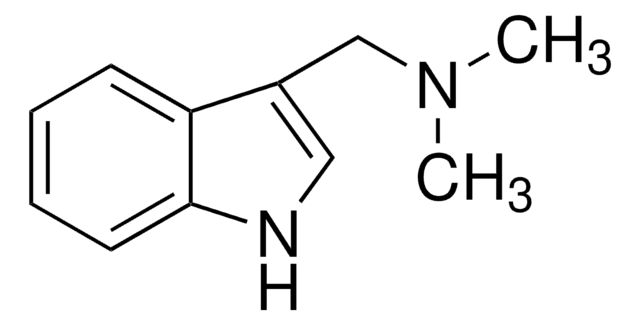
Gramine
97.5%
Sinônimo(s):
3-(Dimethylaminomethyl)indole, Donaxine, NSC 16892
About This Item
Produtos recomendados
Ensaio
97.5%
pf
132-134 °C (lit.)
cadeia de caracteres SMILES
CN(C)Cc1c[nH]c2ccccc12
InChI
1S/C11H14N2/c1-13(2)8-9-7-12-11-6-4-3-5-10(9)11/h3-7,12H,8H2,1-2H3
chave InChI
OCDGBSUVYYVKQZ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
- Dopamine D2 receptor antagonists
- Anti-malarial drugs
- 5-indolyl-Mannich bases
- Proliferation inhibitors
- Inhibitors of human mast cell chymase
- Preparation of DL-tryptophan
- Potential detoxification inhibitors of the crucifer phytoalexin brassinin
- 3-vinylindoles
- Serotonin 5-HT6 receptor ligand templates
- Selective protein kinase c delta (PKCδ) down regulators
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Oral
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
332.6 °F
Ponto de fulgor (°C)
167 °C
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica