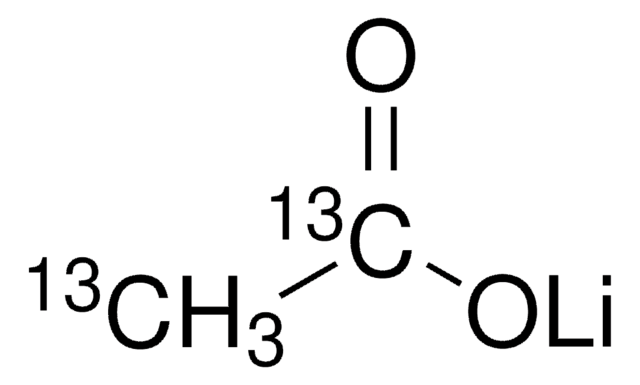
939374
Lithium acetate dihydrate

≥99.9% trace metals basis
Sinônimo(s):
Acetic acid lithium salt, Acetic acid lithium salt dihydrate
About This Item
Produtos recomendados
tipo
(High purity Salts)
Nível de qualidade
Ensaio
≥99.9% trace metals basis
forma
powder or crystals
solid
Impurezas
≤1000 ppm (trace metals analysis)
cor
white to off-white
pH
≤9.5
pf
53-56 °C (lit.)
solubilidade
water: soluble
traços de ânion
chloride (Cl-): ≤20 ppm
sulfate (SO42-): ≤50 ppm
traços de cátion
Al: <100 ppm
Cu: <100 ppm
Fe: <100 ppm
K: <100 ppm
Mg: <100 ppm
Na: ≤50 ppm
Pb: <100 ppm
Zn: <100 ppm
aplicação(ões)
battery manufacturing
cadeia de caracteres SMILES
[Li+].[H]O[H].[H]O[H].CC([O-])=O
InChI
1S/C2H4O2.Li.2H2O/c1-2(3)4;;;/h1H3,(H,3,4);;2*1H2/q;+1;;/p-1
chave InChI
IAQLJCYTGRMXMA-UHFFFAOYSA-M
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Our Lithium acetate dihydrate, with a purity of 99.9% on a trace metals basis, serves as an excellent precursor for batteries and catalysis. Its low trace metals content and anions make it particularly well-suited for these applications.
- Lithium Iron Pyrophosphate (LiFe1.5P2O7) with monoclinic structures was successfully synthesized using Lithium acetate dihydrate in combination with other metal acetates, in a ratio of Li/Fe/P = 1.05:1.5:2, through a wet-chemical method. Maintaining the appropriate lithium concentration is crucial to prevent stoichiometry loss in the final product. This material has found application as a positive electrode in Lithium-ion batteries. Remarkably, the electrode demonstrates excellent incremental capacity, indicating a stable structure during the initial cycle, with redox peaks observed at 3.33 and 3.22 V versus Li0/Li+
- LiMn2O4 films were synthesized on Au foil using the sol-gel and spin-coating techniques, employing Lithium acetate dihydrate and manganese acetate tetrahydrate in a Li/Mn ratio of 1.1/2. The particles used had an average size of approximately 300 nm. To investigate the morphological changes during over-discharging, the EC-HS-AFM technique was utilized. The images captured revealed the presence of wrinkle-like and step-like structures on the particle surface. These structures were attributed to stresses induced by structural distortion during the phase transformation from cubic (LiMn2O4) to tetragonal (Li2Mn2O4). The formation of the Li2Mn2O4 phase was confirmed through ex situ XRD analysis. Furthermore, by analyzing the EC-HS-AFM images, the particle surface area was quantitatively extracted as a function of potential, providing insights into the irreversible expansion/contraction behavior of the particles
- Cobalt-free cathodes, specifically Mg and Zr modified LiNi0.5Mn1.5O4 (LNMO), were synthesized using Lithium acetate dihydrate and other metal acetates via a citric acid sol-gel method. The modifications aimed to improve the electrochemical performance of the cathode, particularly at high temperatures, by limiting Mn dissolution and adjusting interstitial sites. This modification resulted in increased stability of the cathode, extending the cycle life to 1000 cycles at both 25 and 50 °C
Características e benefícios
- Water soluble
- Medium purity (99.9%)
- Low trace metals in ppm level
- Cost effective
- Low Chloride and sulfate levels
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica