930423
CP-alkyne
≥95%
Sinônimo(s):
2,4,6-Trimethyl-1-(methyl((pent-4-yn-1-yloxy)carbonyl)amino)pyridin-1-ium tetrafluoroborate
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C15H21BF4N2O2
Peso molecular:
348.14
Código UNSPSC:
41116164
NACRES:
NA.22
Produtos recomendados
descrição
Application: Chemoproteomics
Nível de qualidade
Ensaio
≥95%
forma
liquid
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
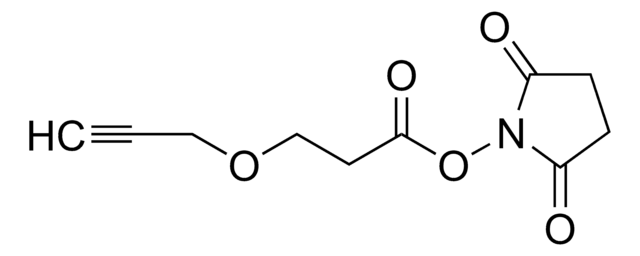
CC1=CC(C)=[N+](N(C(OCCCC#C)=O)C)C(C)=C1.F[B-](F)(F)F
Aplicação
CP-alkyne is a probe that can be used to photochemically label tryptophans. A method was developed using cysteine-reactive compounds including this one to allow for unbiased analysis of proteomic data in quantitative applications (Zanon et al. 2021). The method uses light or heavy labelling with the isotopically labelled desthiobiotin azide (isoDTB) tag for mass spectrometry analysis (Zanon et al. 2020). Analysis then uses the isotopic tandem orthogonal proteolysis activity-based protein profiling (isoTOP-ABPP) workflow (Weerapana et al. 2010, Backus et al. 2016)
Outras notas
1. Profiling the proteome-wide selectivity of diverse electrophiles
2. A quantitative thiol reactivity profiling platform to analyze redox and electrophile reactive cysteine proteomes
3. Ethynylation of Cysteine Residues: From Peptides to Proteins in Vitro and in Living Cells
4. A Chemoproteomic Platform To Assess Bioactivation Potential of Drugs
5. Inhibition of Zinc-Dependent Histone Deacetylases with a Chemically Triggered Electrophile
6. Reversibility of Covalent Electrophile-Protein Adducts and Chemical Toxicity
2. A quantitative thiol reactivity profiling platform to analyze redox and electrophile reactive cysteine proteomes
3. Ethynylation of Cysteine Residues: From Peptides to Proteins in Vitro and in Living Cells
4. A Chemoproteomic Platform To Assess Bioactivation Potential of Drugs
5. Inhibition of Zinc-Dependent Histone Deacetylases with a Chemically Triggered Electrophile
6. Reversibility of Covalent Electrophile-Protein Adducts and Chemical Toxicity
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Profiling the proteome-wide selectivity of diverse electrophiles
Patrick R. A. Zanon ,Fengchao Yu, et al
ChemRxiv : the preprint server for chemistry (2021)
Zarko V Boskovic et al.
ACS chemical biology, 11(7), 1844-1851 (2016-04-12)
Unbiased binding assays involving small-molecule microarrays were used to identify compounds that display unique patterns of selectivity among members of the zinc-dependent histone deacetylase family of enzymes. A novel, hydroxyquinoline-containing compound, BRD4354, was shown to preferentially inhibit activity of HDAC5
De Lin et al.
Chemical research in toxicology, 21(12), 2361-2369 (2009-06-24)
The biotin-tagged electrophiles 1-biotinamido-4-(4'-[maleimidoethylcyclohexane]-carboxamido)butane (BMCC) and N-iodoacetyl-N-biotinylhexylenediamine (IAB) have been used as model electrophile probes in complex proteomes to identify protein targets associated with chemical toxicity. Whereas IAB activates stress signaling and apoptosis in HEK293 cells, BMCC does not. Cysteine
A H El-Khatib et al.
Journal of mass spectrometry : JMS, 52(8), 543-549 (2017-06-04)
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) derivatives are applied in quantitative proteomics owing to their ability to react with different functional groups, to harbor lanthanoides and hence their compatibility with molecular and elemental mass spectrometry. The new DOTA derivatives, namely Ln-MeCAT-Click and Ln-DOTA-Dimedone
Rui Sun et al.
Chemical research in toxicology, 30(10), 1797-1803 (2017-09-30)
Reactive metabolites (RM) formed from bioactivation of drugs can covalently modify liver proteins and cause mechanism-based inactivation of major cytochrome P450 (CYP450) enzymes. Risk of bioactivation of a test compound is routinely examined as part of lead optimization efforts in
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica