901058
J51
Sinônimo(s):
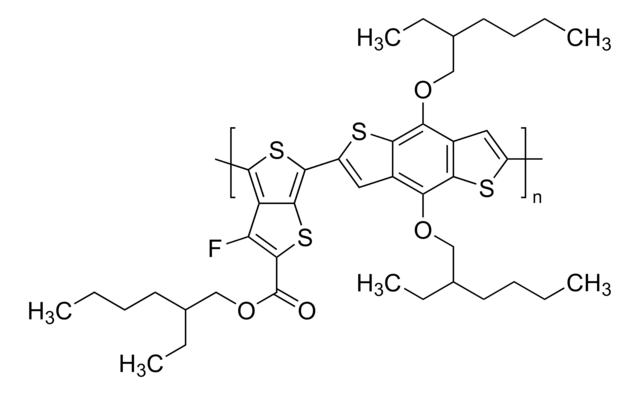
Poly[(5,6-difluoro-2-octyl-2H-benzotriazole-4,7-diyl)-2,5-thiophenediyl[4,8-bis[5-(2-hexyldecyl)-2-thienyl]benzo[1,2-b:4,5-b′]dithiophene-2,6-diyl]-2,5-thiophenediyl]
About This Item
Produtos recomendados
descrição
Band gap: 1.99 eV
Nível de qualidade
forma
solid
peso molecular
Mw 40,000-80,000 by GPC (PS standard)
características do produto alternativo mais ecológico
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
pf
>200 °C
solubilidade
organic solvents: soluble (Soluble in chlorobenzene and dichlorobenzene, Limited solubility in CHCl3)
Energia orbital
HOMO -5.29 eV
LUMO -3.3 eV
PDI
2.0‑3.0
categoria alternativa mais ecológica
Categorias relacionadas
Descrição geral
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
Novel biosensing devices, like bio-FET sensors, offer label-free analysis by detecting ionic and biomolecular charges, crucial for diagnostics and biological research.
Professor Chen (Nankai University, China) and his team explain the strategies behind their recent record-breaking organic solar cells, reaching a power conversion efficiency of 17.3%.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica