About This Item
Fórmula linear:
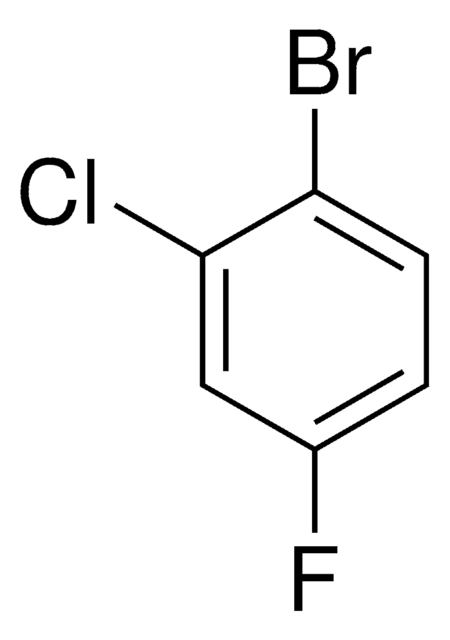
BrC6H3(Cl)F
Número CAS:
Peso molecular:
209.44
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
98%
índice de refração
n20/D 1.556 (lit.)
p.e.
91-92 °C/20 mmHg (lit.)
densidade
1.678 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
Fc1cc(Cl)ccc1Br
InChI
1S/C6H3BrClF/c7-5-2-1-4(8)3-6(5)9/h1-3H
chave InChI
FPNVMCMDWZNTEU-UHFFFAOYSA-N
Descrição geral
1-Bromo-4-chloro-2-fluorobenzene is a polyhalo substituted benzene. It undergoes Suzuki coupling with 2-cyanoarylboronic esters to form the corresponding biphenyls. These biphenyls are the precursors for synthesizing 6-substituted phenanthridines.† The enthalpy of vaporization at boiling point (467.15K) of 1-bromo-4-chloro-2-fluorobenzene is 40.737kjoule/mol.{4}
Aplicação
1-Bromo-4-chloro-2-fluorobenzene may be used in the preparation of benzonorbornadiene derivative. It may also be used as a starting material in the multi-step synthesis of AZD3264, an IKK2 (inhibitor of nuclear factor κ-B kinase-2) inhibitor.
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
198.0 °F - closed cup
Ponto de fulgor (°C)
92.2 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Synthesis of substituted 2-cyanoarylboronic esters.
Lysen M, et al.
The Journal of Organic Chemistry, 71(6), 2518-2520 (2006)
Exploiting the Differential Reactivities of Halogen Atoms: Development of a Scalable Route to IKK2 Inhibitor AZD3264
Murugan A, et al.
Organic Process Research & Development, 18(5), 646-651 (2014)
K C Caster et al.
The Journal of organic chemistry, 66(9), 2932-2936 (2001-04-28)
This report details the synthesis of several benzonorbornadienes by Diels--Alder cycloaddition of cyclopentadiene derivatives with substituted benzyne intermediates, which were generated by low-temperature metal--halogen exchange of halobenzenes. General conditions were developed, allowing synthesis of most benzonorbornadienes described herein at the
Thermophysical Properties of Chemicals and Hydrocarbons, 435-435 (2008)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica